Article

Cutaneous Presentation of Metastatic Salivary Duct Carcinoma
- Author:
- Xintong Wang, MD
- Nikki S. Vyas, MD
- Abdulaziz A. Alghamdi, MD
- Matthew Parker, MD
- Allen Sapadin, MD
- Matthew S. Goldberg, MD
- William Westra, MD
Skin manifestations of metastatic salivary duct carcinoma can be variable, ranging from nodules to erysipelaslike inflammation (also known as...
Article

Postirradiation Pseudosclerodermatous Panniculitis: A Rare Complication of Megavoltage External Beam Radiotherapy
- Author:
- Peter W. Hashim, MD, MHS
- Nikki S. Vyas, MD
- Mohammad-Ali Yazdani Abyaneh, MD
- Matthew S. Goldberg, MD
Postirradiation pseudosclerodermatous panniculitis presents as an erythematous or indurated plaque at a site of prior radiotherapy.
Article

Painful Ulcerating Lesions on the Breast
- Author:
- Nikki S. Vyas, MD
- Teresa Song, MD
- Robert G. Phelps, MD
- Julia Wu, MD
A 36-year-old puerperal woman presented with painful, unilateral, ulcerating breast lesions of 3 months’ duration that developed during pregnancy...
Article
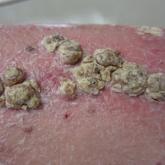
Unilateral Verrucous Psoriasis
- Author:
- Riana D. Sanyal, MD
- Nikki S. Vyas, MD
- Allen N. Sapadin, MD
- Robert G. Phelps, MD
Although psoriasis typically presents in a symmetric distribution, unilateral psoriasis can occur either de novo in younger patients or after...
