Growing the LVAD Numbers
Further growth in LVAD placements will happen on two fronts: broader use in patients at INTERMACS levels 2 and 3, and possibly level 4, where a strong consensus exists for LVAD support; and new evidence to document efficacy and safety in patients with less-severe disease at INTERMACS levels 4, 5, 6, and 7.
For the existing population, it’s a matter of physician and patient awareness. "We need to educate physicians that this is an option," said Dr. Lanfear. "Awareness is lacking. There is a lot of heart failure out there that is underrecognized and underreferred. Neither patients nor their physicians realize how sick they are, and that they are at a high enough risk to justify this."
Furthermore, there are parts of the country where the technology is underused. "In areas without a big center nearby, some physicians may not recognize patients who are sick enough to be LVAD candidates. We are trying to spread the word on who these patients are and when they should be referred."
According to Dr. Teuteberg, major warning signs that should flag patients who are potential LVAD candidates include advanced symptoms, increasing numbers of hospitalizations for heart failure, dwindling responses to ACE inhibitors and beta-blockers, increasing dosages of diuretics, a persistently high serum level of brain natriuretic peptide (BNP) despite good medical treatment, and lack of response to cardiac resynchronization therapy.
Expansion of the evidence base to show LVAD benefits in patients with INTERMACS level 5, 6, or 7 disease depends on a trial just starting, the REVIVE-IT (Randomized Evaluation of VAD Intervention Before Inotropic Therapy) study that’s set to enroll about 100 patients. But at press time, REVIVE-IT had not yet begun, posing doubts about LVAD use in the study’s targeted patients. "We haven’t really demonstrated reproducibly good survival [with LVADs] to compete with medical therapy in level 5 and 6 patients," said Dr. Kirklin. "The FDA put the study on hold while they reflected on that."
Making LVADs Better
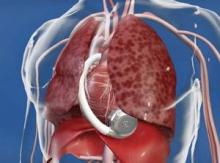
With fast-paced technological advancement, continuous-flow LVADs will continue to evolve and improve. In June, results appeared on a new continuous-flow LVAD, the HeartWare device (Circulation 2012;125:3191-200), and last April an FDA advisory committee recommended that the agency approve the HeartWare LVAD for use as a bridge to (a trial testing the HeartWare LVAD for destination therapy is ongoing).
But no one interviewed for this article anticipates that the HeartWare LVAD will be a major advance. "Fundamentally, the major components and the implant technique are the same for the two devices," the HeartWare and the HeartMate II, said Dr. Slaughter. The HeartWare LVAD is smaller and designed to be placed completely in the pericardial space, but any clinical advantages based on these differences remain to be proved, he said in an interview.
A more meaningful improvement in LVAD design is in the works, and may reach initial clinical testing within a couple of years: a fully implantable LVAD with no transcutaneous drive line, a part that is subject to infection, prevents patients from submerging, and physically and psychologically limits patients by tethering them to equipment. "If there were one thing that could make a dramatic difference, it would be getting rid of the drive line. That is the Holy Grail for the field," said Dr. Bailey.
When a fully implantable LVAD becomes available for routine use, it will complete the LVAD revolution and help device therapy for advanced heart failure reach its full potential.
Dr. Lanfear has received research support and has received honoraria as a speaker for Thoratec, the company that markets the HeartMate II, and has received research support from HeartWare, the company developing the HeartWare LVAD. Dr. Slaughter has had contracts for services to Thoratec and HeartWare. Dr. Kirklin, Dr. Bailey, Dr. Teuteberg, and Dr. Taylor said that they had no disclosures.
*CORRECTION 8/10/12: The credit for the photo with the caption "The next LVAD in line, HeartWare, is smaller and dwells in the pericardial space" was misstated and should have been ©2012 HeartWare International, Inc.