An implantable device that stimulates the upper airway nerves and muscles produced long-term, clinically meaningful reductions in the severity of obstructive sleep apnea in an industry-sponsored study reported in the New England Journal of Medicine.
One year after implantation, patients showed a 68% reduction in scores on the Apnea-Hypopnea Index (AHI) and a 70% reduction in scores on the Oxygen Desaturation Index (ODI), as well as subjective improvements in daytime sleepiness and quality of life. The magnitude of these benefits was similar to that reported for continuous positive airway pressure (CPAP) therapy and superior to that reported for uvulopalatopharyngoplasty, said Dr. Patrick J. Strollo Jr., FCCP, of the department of otolaryngology, University of Pittsburgh Medical Center and his associates in the STAR (Stimulation Therapy for Apnea Reduction) trial group.
Upper airway stimulation using implanted electrodes to stimulate the hypoglossal nerve on one side of the neck "has been developed as a possible treatment option" for moderate to severe obstructive sleep apnea "and has shown promise in feasibility trials," the investigators noted.
For their study, designed in collaboration with the sponsor (Inspire Medical Systems) and the Food and Drug Administration, 126 patients who couldn’t tolerate CPAP therapy underwent surgical implantation of the device and were followed for 1 year. Otolaryngologists at 22 academic and private medical centers performed the surgery, which took a median of 140 minutes (range, 65-360 minutes).
Most (83%) of the participants were men. The mean age was 55 years (range, 31-80 years), and the mean body mass index was 28.4 kg/m2 (range, 18.4-32.5). Twenty-two of these patients (17%) had undergone uvulopalatopharyngoplasty, which had not corrected their obstructive sleep apnea.
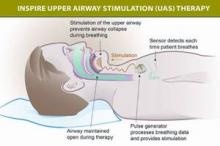
The device includes a neurostimulator implanted in the intercostal muscles in the right mid-infraclavicular region, with one lead threaded upward inside the patient’s neck that is attached to three stimulation electrodes. The electrodes can be placed in a variety of configurations on the ipsilateral hypoglossal nerve, which, when stimulated, pushes the tongue forward and prevents the upper-airway muscles from collapsing and causing inspiratory flow obstruction.
The device also has a second, sensing lead tunneled between the internal and external intercostal muscles on the ipsilateral side to detect ventilatory effort during sleep, so that the stimulation of the hypoglossal nerve can be synchronized with the patient’s breathing.
The primary outcome of the study was the change in severity of obstructive sleep apnea, as measured by scores on the AHI and the ODI, at 12 months. The median AHI score decreased 68%, from 29.3 events per hour to 9.0 events per hour. The median ODI score dropped 70%, from 25.4 events per hour to 7.4 events <[lb]>per hour, the investigators said (N. Engl. J. Med. 2014 [doi:10.1056/<[lb]>NEJMoa1308659]).
Two-thirds of the participants showed a reduction of at least 50% in AHI score, and three-quarters showed a reduction of at least 25% in ODI score. And the median percentage of sleep time spent with poor oxygen saturation (less than 90%) declined from 5.4% to 0.9%.
In addition, patients’ scores on the Functional Outcomes of Sleep Questionnaire, which measures disease-specific quality of life, showed clinically meaningful improvement. And scores on the Epworth Sleepiness Scale normalized.
In the final, "challenge," phase of this study, the first 46 consecutive patients who had responded to this treatment at 1 year were randomly assigned to either continue it (turn the devices on at night) or to discontinue it (turn the devices off at night) for 1 more week. This challenge demonstrated that the improvements in OSA were in fact from the use of the hypoglossal-stimulation device, as sleep apnea relapsed in the patients who discontinued treatment.
There were no serious procedural complications, no rehospitalizations, and no infections. Two patients developed serious device-related adverse events, for an overall rate of less than 2%. In both cases, the device caused discomfort that was resolved by a second surgery to reposition it. Another 33 serious adverse events were considered to be unrelated to the implantation procedure or the device .
Nonserious adverse events – including sore throat from intubation during the procedure, pain at the incision sites, and muscle soreness – occurred in 88% of the study subjects. Nonserious events related to the device included discomfort during electrostimulation, reported by 40% of patients, and tongue soreness, reported by 21%. These resolved as the patients became acclimated to the device or after the device was reprogrammed to adjust the stimulation.