Evidence From Brodalumab Studies
Intensive efforts have been made to assess the effect of brodalumab, a fully human anti–IL-17RA monoclonal antibody shown to be efficacious in the treatment of moderate to severe plaque psoriasis, on symptoms of depression and to understand the incidence of SIB among patients receiving brodalumab in clinical trials.22-27
Depression and Anxiety in Studies of Brodalumab
To examine the effects of brodalumab on symptoms of depression, the HADS questionnaire28 was administered to patients in 1 of 3 phase 3 clinical trials of brodalumab.23 A HADS score of 0 to 7 is considered normal, 8 to 10 is mild, 11 to 14 is moderate, and 15 to 21 is severe.23 The HADS questionnaire was administered to evaluate the presence and severity of depression and anxiety symptoms at baseline and at weeks 12, 24, 36, and 52.25 This scale was not used in the other 2 phase 3 studies of brodalumab because at the time those studies were initiated, there was no indication to include mental health screenings as part of the study protocol.
Patients were initially randomized to placebo (n=220), brodalumab 140 mg every 2 weeks (Q2W; n=219), or brodalumab 210 mg Q2W (the eventual approved dose; n=222) for 12 weeks.23 At week 12, patients initially randomized to placebo were switched to brodalumab through week 52. Patients initially randomized to brodalumab 210 mg Q2W were re-randomized to either placebo or brodalumab 210 mg Q2W.23 Depression and anxiety were common at baseline. Based on HADS scores, depression occurred among 27% and 26% of patients randomized to brodalumab and placebo, respectively; anxiety occurred in 36% of patients in each group.22 Among patients receiving brodalumab 210 mg Q2W from baseline to week 12, HADS depression scores improved in 67% of patients and worsened in 19%. In contrast, the proportion of patients receiving placebo whose depression scores improved (45%) was similar to the proportion whose scores worsened (38%). Hospital Anxiety and Depression Scale anxiety scores also improved more often with brodalumab than with placebo.22
Furthermore, among patients who had moderate or severe depression or anxiety at baseline, a greater percentage experienced improvement with brodalumab than placebo.23 Among 30 patients with moderate to severe HADS depression scores at baseline who were treated with brodalumab 210 mg Q2W, 22 (73%) improved by at least 1 depression category by week 12; in the placebo group, 10 of 22 (45%) improved. Among patients with moderate or severe anxiety scores, 28 of 42 patients (67%) treated with brodalumab 210 mg Q2W improved by at least 1 anxiety category compared to 8 of 27 (30%) placebo-treated patients.23
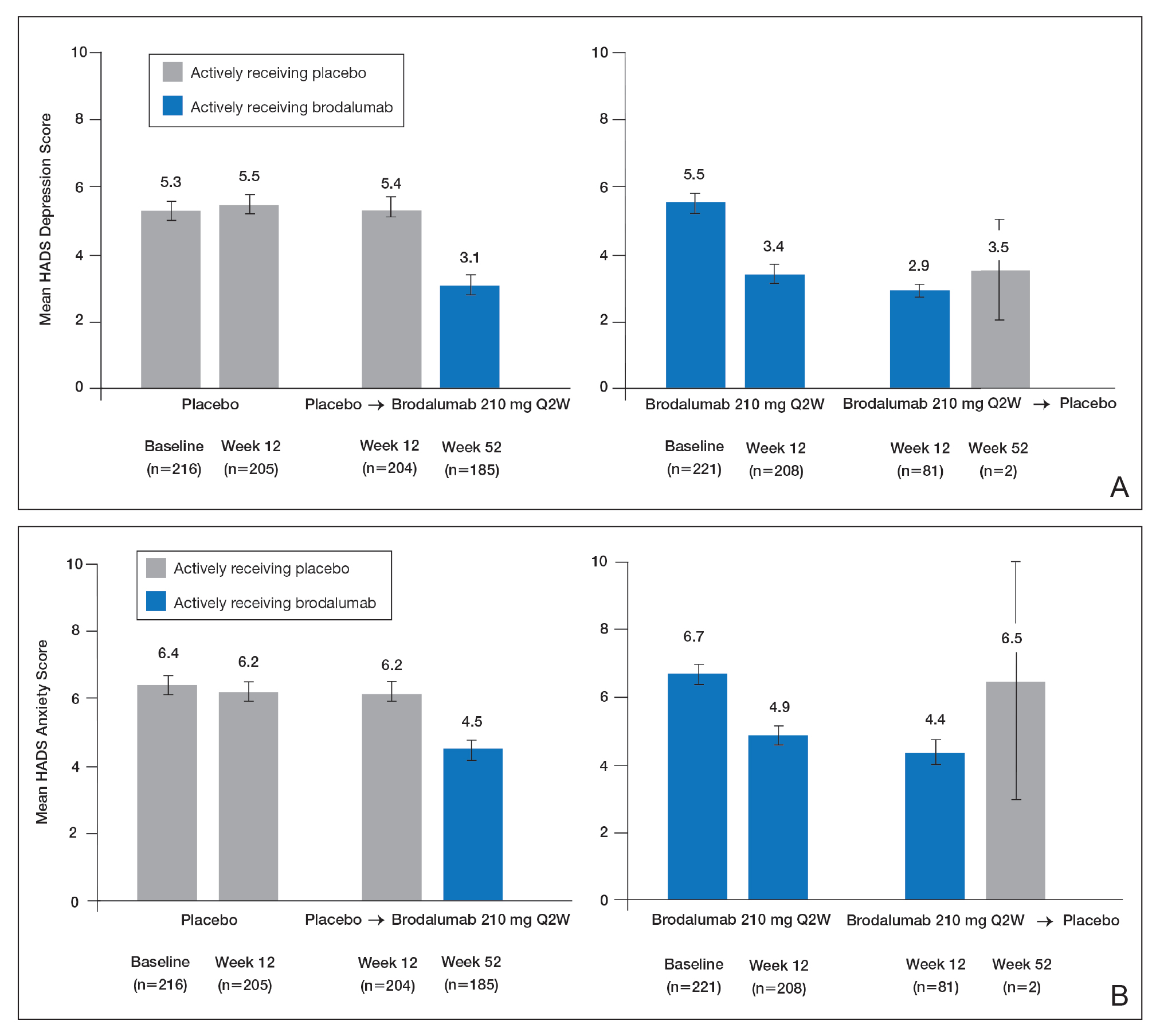
Over 52 weeks, HADS depression and anxiety scores continued to show a pattern of improvement among patients receiving brodalumab vs placebo.25 Among patients initially receiving placebo, mean HADS depression scores were unchanged from baseline (5.3) to week 12 (5.5). After patients were switched to brodalumab 210 mg Q2W, there was a trend toward improvement between week 12 (5.4) and week 52 (3.1). Among patients initially treated with brodalumab 210 mg Q2W, mean depression scores fell from baseline (5.5) to week 12 (3.4), then rose again between weeks 12 (2.9) and 52 (3.5) in patients switched to placebo (Figure, A). The pattern of findings was similar for HADS anxiety scores (Figure, B).25 Overall, brodalumab treatment appears to improve symptoms of depression and anxiety in patients being treated for psoriasis. This finding is consistent with the effects reported for other biologic therapies previously discussed.