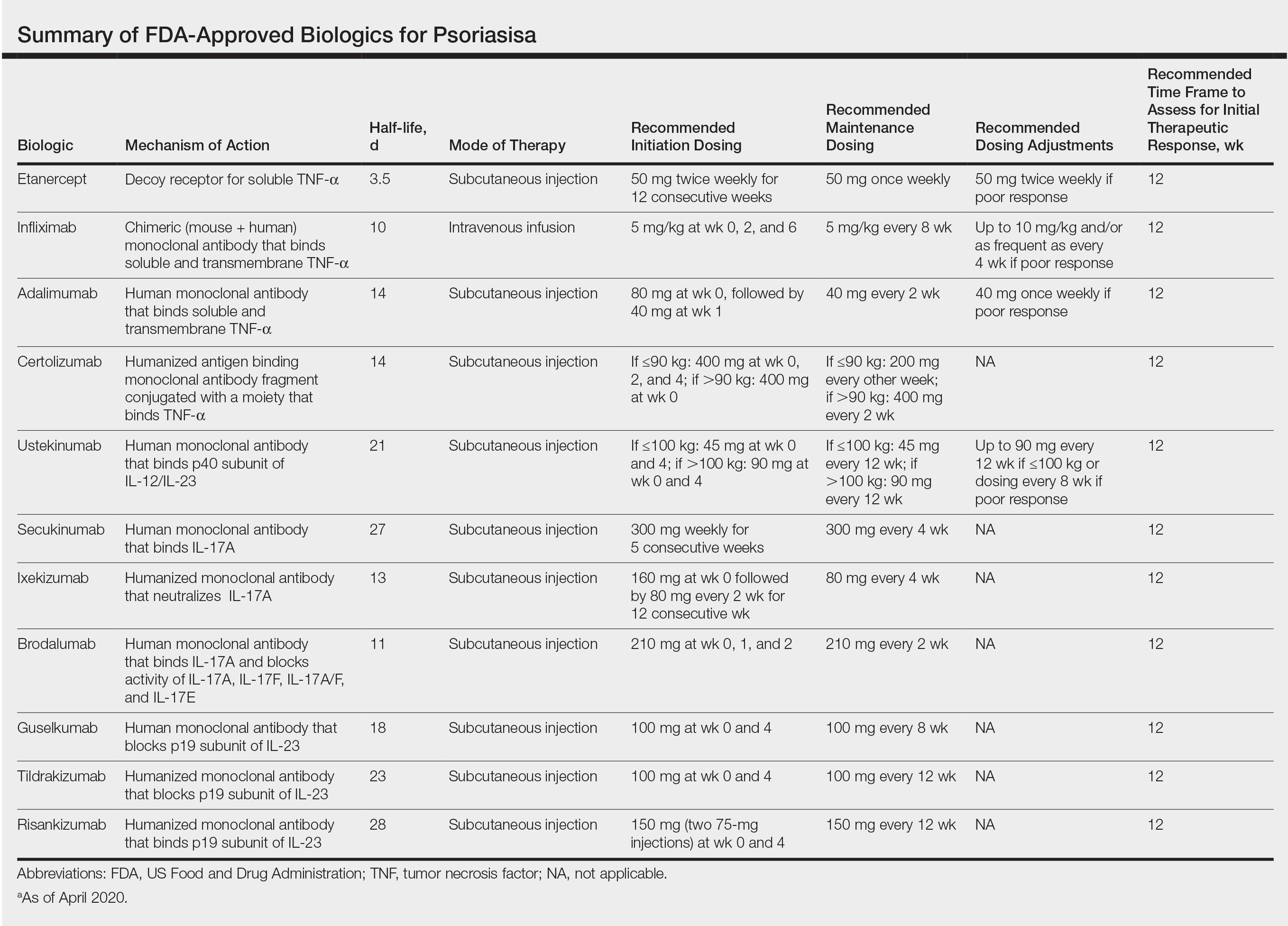
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.
Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.