Adoption of emerging definitions
The document adopts the emerging characterization of HFrEF by a left ventricular ejection fraction (LVEF) up to 40%.
And it will leverage an expanding evidence base for medication in a segment of patients once said to have HF with preserved ejection fraction (HFpEF), who had therefore lacked specific, guideline-directed medical therapies. Now, patients with an LVEF of 41%-49% will be said to have HF with mildly reduced ejection fraction (HFmrEF), a tweak to the recently introduced HF with “mid-range” LVEF that is designed to assert its nature as something to treat. The new document’s HFmrEF recommendations come with various class and level-of-evidence ratings.
That leaves HFpEF to be characterized by an LVEF of 50% in combination with structural or functional abnormalities associated with LV diastolic dysfunction or raised LV filling pressures, including raised natriuretic peptide levels.
The definitions are consistent with those proposed internationally by the ESC-HFA, the Heart Failure Society of America, and other groups in a statement published in March.
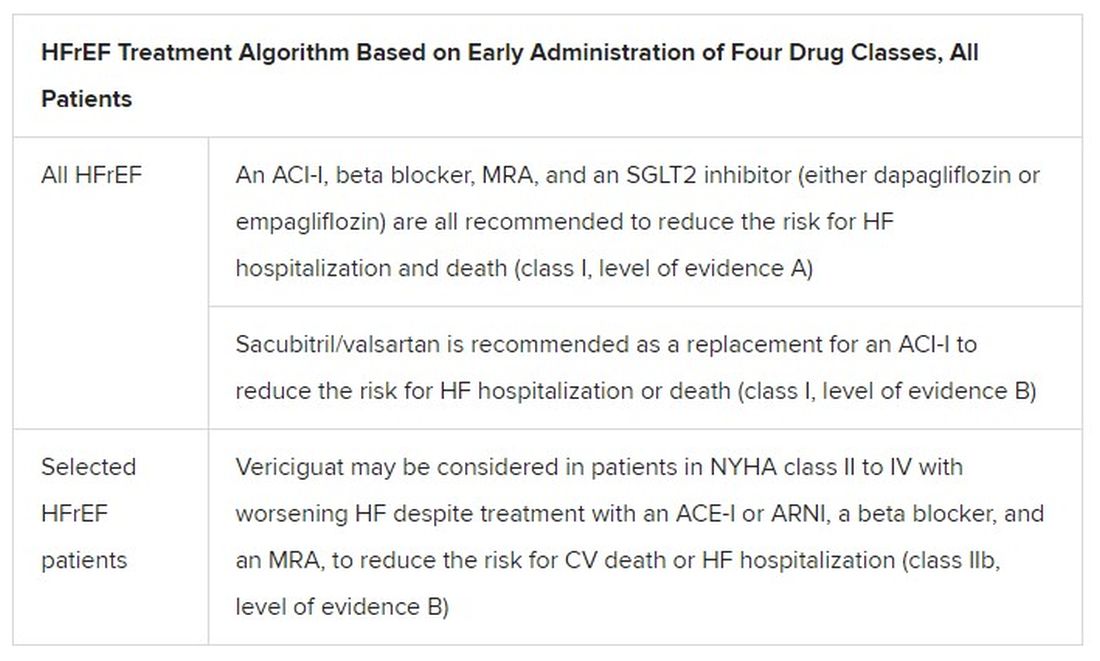
Expanded HFrEF med landscape
Since the 2016 ESC guideline on HF therapy, Dr. McDonagh said, “there’s been no substantial change in the evidence for many of the classical drugs that we use in heart failure. However, we had a lot of new and exciting evidence to consider,” especially in support of the SGLT2 inhibitors as one of the core medications in HFrEF.
The new data came from two controlled trials in particular. In DAPA-HF, patients with HFrEF who were initially without diabetes and who went on dapagliflozin (Farxiga, AstraZeneca) showed a 27% drop in cardiovascular (CV) death or worsening-HF events over a median of 18 months.
“That was followed up with very concordant results with empagliflozin [Jardiance, Boehringer Ingelheim/Eli Lilly] in HFrEF in the EMPEROR-Reduced trial,” Dr. McDonagh said. In that trial, comparable patients who took empagliflozin showed a 25% drop in a primary endpoint similar to that in DAPA-HF over the median 16-month follow-up.
Other HFrEF recommendations are for selected patients. They include ivabradine, already in the guidelines, for patients in sinus rhythm with an elevated resting heart rate who can’t take beta-blockers for whatever reason. But, Dr. McDonagh noted, “we had some new classes of drugs to consider as well.”
In particular, the oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo) emerged about a year ago from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization. In the trial with more than 5,000 patients, treatment with vericiguat atop standard drug and device therapy was followed by a significant 10% drop in risk for CV death or HF hospitalization.
Available now or likely to be available in the United States, the European Union, Japan, and other countries, vericiguat is recommended in the new guideline for VICTORIA-like patients who don’t adequately respond to other indicated medications.
Little for HFpEF as newly defined
“Almost nothing is new” in the guidelines for HFpEF, Dr. Metra said. The document recommends screening for and treatment of any underlying disorder and comorbidities, plus diuretics for any congestion. “That’s what we have to date.”
But that evidence base might soon change. The new HFpEF recommendations could possibly be up-staged at the ESC sessions by the August 27 scheduled presentation of EMPEROR-Preserved, a randomized test of empagliflozin in HFpEF and – it could be said – HFmrEF. The trial entered patients with chronic HF and an LVEF greater than 40%.
Eli Lilly and Boehringer Ingelheim offered the world a peek at the results, which suggest the SGLT2 inhibitor had a positive impact on the primary endpoint of CV death or HF hospitalization. They announced the cursory top-line outcomes in early July as part of its regulatory obligations, noting that the trial had “met” its primary endpoint.
But many unknowns remain, including the degree of benefit and whether it varied among subgroups, and especially whether outcomes were different for HFmrEF than for HFpEF.