Castleman disease (CD) is a rare nonclonal lymphoproliferative disorder, also known as angiofollicular lymph-node hyperplasia or giant node hyperplasia. It was first reported in 1954 and in 1956 described by Benjamin Castleman, MD, in a case series of localized mediastinal lymph-node hyperplasia.1 Unicentric Castleman (UCD) disease presents as a localized disease affecting a single lymph node/lymph node chain. Multicentric Castleman disease (MCD) is a more widespread or generalized disease (Table 1). About 4,000 to 6,000 new cases of CD are diagnosed per year of which about 20% to 25% cases are MCD. The estimated incidence rate for CD has recently been calculated as 21 to 25 per million person-years, or about 6,000 new cases annually.2
The clinical presentation of CD often overlaps with autoimmune, infectious, or other malignant diseases. The diagnosis is confirmed by a biopsy of the affected lymph-node tissue. Interleukin-6 (IL-6) and a viral analog of IL-6 play major role in the pathogenesis by stimulating a widespread inflammatory response that results in systemic manifestations. It is often associated with HIV and human herpesvirus-8 (HHV-8) infections. Castleman disease is histologically characterized into the hyaline vascular variant, the plasma-cell variant, and the mixed form. The plasmablastic variety is associated with HIV and HHV-8 infections. The prognosis ranges from good in UCD (91% overall survival [OS] at 5 y) to poor in MCD (65% OS at 5 y).3
Treatment options range from local surgical excision to systemic treatments. Newer therapies include monoclonal antibodies against both IL-6 and CD20 and a few other targets in the inflammatory cascade. This article discusses the updated approach to diagnosis and management of CD.
Unicentric Castleman Disease
Unicentric CD is more common than MCD, presents as a localized lymph node or chain involvement, and is generally diagnosed in the third or fourth decade of life but has been reported in children. The presenting symptoms of UCD vary by site. It presents as nontender lymphadenopathy when confined to peripheral lymph nodes, whereas respiratory symptoms or bowel obstruction may be seen with lymphadenopathy in the chest/mediastinum, neck, or abdomen. The systemic
symptoms, such as fever, night sweats, and weight loss, are uncommon.
Dysplastic follicular dendritic cells characterize UCD. Histologically, it is usually classified as hyaline vascular disease with the follicles comprising small lymphocytes
and dendritic cells forming concentric rings with prominent vascularity.4,5 No association with HIV or HHV-8 has been seen.
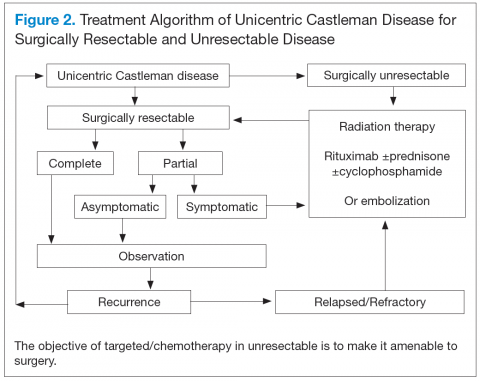
Unicentric CD is often amenable to resection, and a complete cure can be achieved.6 Partial resection may be attempted when complete resection is not possible. Radiation therapy is offered for unresectable disease.7 In patients who are not candidates for any intervention, close long-term follow-up is recommended unless patients are symptomatic, in which case systemic treatment should be considered.
Multicentric Castleman Disease
The more widespread MCD is generally diagnosed in the fifth or sixth decade of life. It is more aggressive than UCD and presents a wide spectrum of symptoms and abnormal laboratory findings (Table 2).8
It is histologically classified into (a) plasmablastic or HHV-8 associated: It is often seen in patients with MCD infected with HIV, which can give rise to large B-cell lymphoma, known as HHV-8 plasmablastic lymphoma9; (b) plasmacytic variant has marked paracortical plasmacytosis with retained nodal architecture10; and (c) mixed MCD has abundant plasma cells with features similar to those of the hyaline-vascular variant.
Most patients with HIV-associated MCD are co-infected with HHV-8. The HHV-8 infection is also present in about 50% of HIV-negative cases.11 The incidence of HIVassociated MCD is increasing in the highly active antiretroviral therapy (HAART) era secondary to improved survival of patients infected with HIV.12 To diagnose active HIV MCD, the French Agence Nationale de Recherche sur le SIDA 117 CastlemaB trial group has described criteria based on the clinical symptoms, including fever, a raised serum C-reactive protein > 20 mg/L without any other cause, and 3 of 12 additional clinical findings described as peripheral lymphadenopathy, splenomegaly, ascites, edema, pleural effusion, cough, nasal obstruction, xerostomia, rash, central neurologic symptoms, jaundice, and autoimmune hemolytic anemia.13 The reported 2-year survival of patients who are HIV-negative is 97% compared with HIV-positive cases at 67%.14
Idiopathic MCD is diagnosed when there is no evidence of any underlying infectious, autoimmune, and neoplastic process.15
Patients with MCD are at an increased risk of developing non-Hodgkin and Hodgkin lymphoma, Kaposi sarcoma, primary effusion lymphoma, and follicular dendritic
cell sarcoma. POEMS (peripheral neuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome and paraneoplastic disease, such as paraneoplastic pemphigus myasthenia gravis, may be commonly diagnosed concurrently or sequentially with MCD.16-20
The disease course of MCD ranges from indolent to rapidly progressive, and its 5-year OS is about 65%. When associated with POEMS syndrome, the 5-year survival was estimated to be 90% with the osteosclerotic variant and 27% without osteosclerotic lesions.3 Treatment options for MCD include systemic chemotherapy, including antiviral therapy for HHV-8 positive and HAART for HIV positive and newer monoclonal antibody therapies targeting CD20 or IL-6.
Pathophysiology
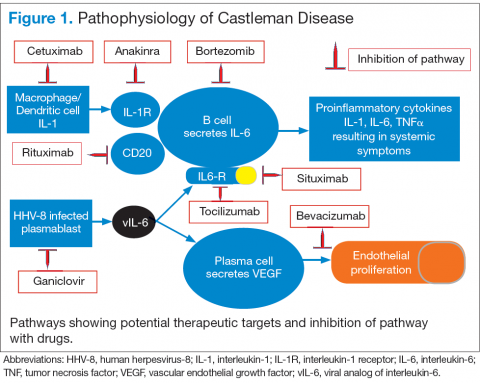
Interleukin-6 plays an important role for inflammation in both UCD and MCD (Figure 1). There is dysregulation and overproduction of IL-6, which further stimulates the production of acute-phase reactants, resulting in various systemic manifestations.15,21,22 There is increased expression of IL-1 and IL-6, upregulation of IL-6 secondary to interaction of IL-1 with nuclear factor-kappa B (NF-kappa B), thus stimulating B-cell proliferation. IL-6 binding to IL-6 receptor (IL6-R) results in downstream activation of transcription Janus kinases/signal transducers and activators of the transcription pathway. This promotes the transcription of genes encoding the acute-phase reactant proteins. Hence, interfering with IL-6 transduction by blocking downstream signals are potential therapeutic targets. The mitogen-activated protein kinase cascade, the rapidly accelerated fibrosarcoma kinases, and the overexpression of the endothelial growth factor receptor (EGFR), all contribute to disease pathogenesis by promoting increased B-cell proliferation and vascular EGFR mediated angiogenesis. 23,24
In HHV-8–associated MCD, the virus replicates within lymph node plasmablasts, causing increased production of viral IL-6 analog, human IL-6, and other proinflammatory proteins resulting in B-cell and plasma-cell proliferation, increased vascular endothelial growth factor secretion and angiogenesis.25,26 The HHV-8–infected plasmablasts are marked by variable expression of CD20, and therefore, anti-CD20 is also shown to be an effective treatment. The calmodulin/calcineurin nuclear factor assists in the proliferation of HHV-8, thereby making calcineurin another potential target for the antiviral proliferation.27
Staging
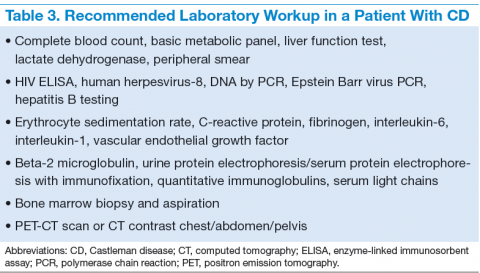
The treatment decisions and prognosis for patients with CD is based on the clinical and histologic staging. The initial workup includes but is not limited to routine laboratory evaluation, imaging, and HIV and HHV-8 testing (Table 3). Routine tests of the levels of cytokines are not recommended. Other relevant tests for known disease associations should be obtained when relevant.
Treatment
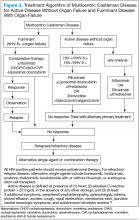
Better understanding of the disease process in CD has helped to identify potential therapeutic targets (Figures 2 and 3).
For UCD, surgery is the mainstay of treatment.4,28,29 In surgically unresectable cases, radiation therapy is helpful for local disease control. Alternatively, neoadjuvant
chemotherapy and rituximab are used. Corticosteroids are generally used to treat acute exacerbations and as adjuncts to chemotherapy.
For MCD, the treatment approach depends on the HIV and HHV-8 status of the patient. For patients with HHV-8 infection, both with and without HIV co-infection, antiviral agents, such as ganciclovir, foscarnet, or cidofovir, have shown in vitro activity against HHV-8 but with limited clinical success.30 In patients infected with HIV, the aim of treating with HAART is to control the disease, prevent opportunistic infections, and improve tolerance to chemotherapy.31-33 Rituximab with or without chemotherapy is the standard treatment approach. The additional chemotherapeutic agents are used depending on the presence or absence of organ failure. This approach has improved the OS in HIV-associated MCD.34,35 Treatment with HAART does not decrease the risk of relapse in HIV MCD; therefore, the role of rituximab and antiherpesvirus agents as maintenance therapy has been explored.36 In patients who fail to respond to or relapse rapidly following rituximab monotherapy, the use of either single-agent chemotherapy with or without rituximab or antiherpesvirus therapy with high-dose zidovudine and valganciclovir is recommended.37
The cytotoxic chemotherapy with single agents, such as etoposide, vinblastine, cyclophosphamide, cladribine, chlorambucil, and liposomal doxorubicin, has been used with limited success.22 The combination chemotherapy with cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) or cyclophosphamide/vincristine/
prednisone (CVP) without rituximab has been shown to achieve durable remissions. Corticosteroids are usually administered as an initial adjunct to chemotherapy or for acute exacerbations. In patients with MCD, regardless of HIV status, the interferon therapy was found to achieve long-term remission.38,39 The interferon therapy
exerts antiviral effects via downregulation of the IL-6R and inhibition of HHV-8 replication. For patients in remission, maintenance therapy with oral valganciclovir is promising.40
Immunomodulators & Targeted Therapies
For unresectable UCD or MCD with organ failure or relapse, the use of alternativesingle-agent or combination chemotherapies with or without rituximab is recommended. Thalidomide has shown some success, probably secondary to disruption of IL-6 production.41 In cases of progression following second-line therapy, bortezomib, antiherpesvirus therapies, or IL-6–directed therapy with siltuximab or tocilizumab should be considered.
Rituximab is a monoclonal chimeric antibody that targets CD20 on B cells, thus leading to B-cell lymphodepletion via activating complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. As monotherapy, it has been shown to achieve 2-year progression-free survival in 80% of patients.42 In patients with MCD who are HIV positive, rituximab with and without chemotherapy has shown improved overall and disease-free survival of 70% to 80% at 2 years.43
Siltuximab is a chimeric human-mouse monoclonal antibody to IL-6 that has been approved for treatment of patients with MCD who are both HIV negative and HHV-8 negative.44-46 Tocilizumab targets the IL-6R. The antibody has shown improvement in a study in HIVseronegative adults with MCD.47,48
Bortezomib is a proteasome inhibitor that inhibits the NF-kappa B pathway, which induces the expression of numerous proinflammatory proteins, including IL-6. It is recommended for relapsed or refractory disease.49,50
Anakinra is a recombinant IL-1R antagonist that blocks IL-1 effects and controls disease by decreasing IL-6 production.51
Conculsion
There has been significant progress in disease diagnosis and management as more information is available about the incidence, clinical presentation, and pathophysiology of CD. The understanding of the disease pathogenesis and biology has helped to discover multiple potential therapeutic targets. Successful treatment has been achieved through targeting HHV-8 replication, CD20, and IL-6 and anti– IL-6R antibodies. Although surgical resection continues to be the
standard of therapy for UCD, the management of MCD and relapsed or refractory disease continues to evolve. Exploration of various treatment strategies in different clinical presentations is warranted.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S.
Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information
for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to
patients.