Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
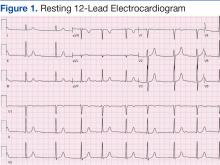
As a result, CABG was performed with a LIMA conduit to the left anterior descending artery (LAD) and a saphenous venous graft to the posterior descending artery. On physical examination, the patient was comfortable with a heart rate of 70 bpm, blood pressure of 120/80 mm Hg (measured on right arm and no significant difference in blood pressure was reported on the left side).Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
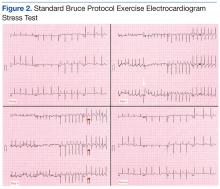
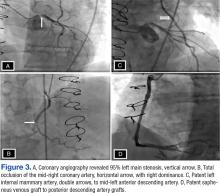
The patient was able to exercise for 5 minutes 20 seconds on a standard Bruce protocol, reaching a heart rate of 169 bpm (109% of maximum predicted heart rate) and achieving 7.1 metabolic equivalents. The test was stopped because of dyspnea. The electrocardiogram showed isolated premature ventricular premature complexes (stage 2, white arrow) and 2- to 3-mm horizontal ST depression at peak exercise (stage III, double arrows) in V4 to V6 that quickly became upsloping and then resolved at rest (Figure 2). Otherwise, the patient had an uneventful recovery period.Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
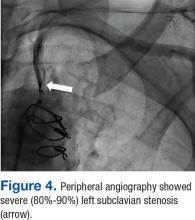
(Supplemental videos 1, 2, 3, and 4). Peripheral angiography showed severe (80%-90%) left subclavian stenosis proximal to the takeoff of the LIMA graft with retrograde flow into the entire LIMA during contrast injection (Figure 4) (Supplemental video 5). Given these findings, the patient was diagnosed with CSSS and was referred for intervention.The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.