Methods
This study involved a qualitative description of individual POC interventions as well as a retrospective data analysis that examined the clinical impact of these interventions during the study period from April 1, 2012 to September 30, 2015. This study was reviewed and approved by the Medical University of South Carolina Institutional Review Board and the RHJVAMC Research and Development Committee.
Setting
The RHJVAMC is a tertiary care teaching hospital with primary and specialized outpatient services that are provided at the main medical center in Charleston, South Carolina, and at 6 community-based outpatient clinics (CBOCs) located throughout southeastern South Carolina and parts of Georgia. Primary care is delivered by patient aligned care teams (PACTs) based on the patient-centered medical home model.28 The PACT consists of a primary care provider (PCP) who is aided by dedicated nursing, pharmacy, and mental health care providers. In most cases, COT is prescribed and managed in the PACT setting. At the time of this initiative, a broad range of specialty services were available, including a multidisciplinary pain management team, orthopedics, and physical medicine and rehabilitation. In 2012, about 55,000 patients were enrolled and received care at RHJVAMC. The POC interventions were carried out at all clinic sites.
Patients
The study population included all patients prescribed COT at RHJVAMC during the study period. A patient was considered to be prescribed COT if at least 1 opioid-containing medication was dispensed to the patient in a selected fiscal quarter during the study period and the total cumulative supply of opioid-containing medications was ≥ 90 days for both the selected quarter and the prior quarter.
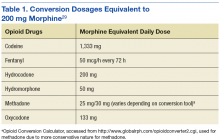
Furthermore, a high-risk COT subpopulation included any patient who satisfied either of the following criteria: (1) Receipt of outpatient prescription(s) for opioid-containing medication(s) (including tramadol) and a benzodiazepine derivative in the same fiscal quarter; patients were included in this subpopulation regardless of whether they met COT criteria; (2) Receipt of outpatient prescription(s) for opioid-containing medication(s) with at least 1 instance in which the MEDD was ≥ 200 mg in the designated quarter (Table 1). The MEDD was calculated for each fill in the fiscal quarter using the following equation:
If medication fills were within 3 days of each other, the prescriptions were considered to be taken together and the MEDD was summed.
Intervention Descriptions
The primary aim of this study was to qualitatively describe each intervention implemented by the POC. The POC monthly meeting minutes were recorded and reviewed for the study period, and descriptive information regarding each intervention was extracted. Extracted information included implementation date(s), the responsible POC member, and a general description of each intervention. Interventions were then categorized as informatics tool, targeted patient intervention, provider education, or patient education.
Impact of Interventions on Monitoring
In order to characterize the impact of POC interventions on appropriate monitoring of COT, the electronic medical record (EMR) of each patient satisfying COT criteria was queried for the presence of an annual urine drug screen (UDS) result and a note in the chart signaling that a prescription drug monitoring program (PDMP) review had been performed. The authors defined appropriate UDS monitoring and PDMP review as the presence of a UDS result and a PDMP review note in the EMR in the year prior to the query date.
Prior to the start of the POC interventions, 4.1% of RJVAMC patients had an annual PDMP review and 47.8% had an annual UDS. Although more frequent UDS results and PDMP reviews are appropriate in most cases, yearly monitoring was considered by the POC to be a reasonable initial goal. The percentage of veterans receiving COT who had received appropriate monitoring for each measure was collected for each fiscal quarter during the study period. In addition, the difference between the initial and final fiscal quarter during the study period was calculated for each measure.
Impact of Interventions on High-Risk Opioid Prescribing
To assess the impact of POC interventions on appropriate management of high-dose COT, clinical variables were collected for patients who were prescribed high-dose COT and received targeted intervention in the form of a pain clinic e-consult. These variables were MEDD, presence of annual UDS, presence of annual PDMP review, and active BZD prescription. Each variable was assessed on the date of intervention (e-consult submission) and at 6 months postintervention. Changes in each clinical variable between baseline and at 6 months postintervention were then evaluated.
Data Sources
All patient data were obtained from the VHA Corporate Data Warehouse (CDW). The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999 to the present. Population-level data were obtained from the Opioid Safety Initiative Master Dashboard National Report where available. Data not contained in this national dashboard were obtained through local data extractions from the CDW.