A majority of sites’ (63.5%) quality reports represented individual provider data, whereas fewer provided quality reports for physician groups (22.9%) or for the entire facility (40.6%). Provider quality information was de-identified in 43.8% of reporting sites’ quality reports and identifiable in 37.5% of reporting sites’ quality reports. A majority of sites (74.0%) reported that the local gastroenterology section chief or quality manager has access to the quality reports. Fewer sites reported providing data to individual endoscopists (44.8% for personal and peer data and 32.3% for personal data only). One site (1%) responded that quality reports were available for public access. Survey respondents also were asked to provide the estimated time (hours required per month) to collect the data for quality metrics. Of 75 respondents providing data for this question, 28 (29.2%) and 17 (17.7%), estimated between 1 to 5 and 6 to 10 hours per month, respectively. Ten sites estimated spending between 11 to 20 hours, and 7 sites estimated spending more than 20 hours per month collecting quality metrics. A total of 13 respondents (13.5%) stated uncertainty about the time burden.
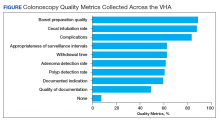
As shown in the Figure, numerous quality metrics were collected across sites with more than 80% of sites collecting information on bowel preparation quality (88.5%), cecal intubation rate (87.5%), and complications (83.3%). A majority of sites also reported collecting data on appropriateness of surveillance intervals (62.5%), colonoscopy withdrawal times (62.5%), and ADRs (61.5%). Seven sites (7.3%) did not collect quality metrics.
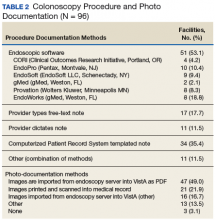
Information also was collected on colonoscopy procedure documentation to inform future efforts at standardization. A small majority (53.1%) of sites reported using endoscopic software to generate colonoscopy procedure documentation. Within these sites, 6 different types of endoscopic note writing software were used to generate procedure notes (Table 2).
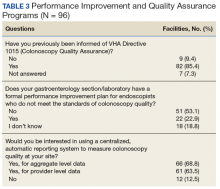
Remaining sites used a variety of methods to generate procedure notes, including typing free-text notes (17.7%) and dictation (11.5%); 35.4% of sites use a template note in the Computerized Patient Record System. Sites also used a variety of methods for photo documentation in the electronic medical record (eg, pictures of cecal intubation and polyps).Most sites (85.4%) were aware of VHA Directive 1015 recommendations for colonoscopy quality assurance programs. A significant majority (89.5%) of respondents also indicated interest in a centralized automatic reporting system to measure and report colonoscopy quality in some form, either with aggregate data, provider data, or both (Table 3).
Discussion
This survey on colonoscopy quality assurance programs is the first assessment of the VHA’s efforts to measure and report colonoscopy quality indicators. The findings indicated that the majority of VA sites are measuring and reporting at least some measures of colonoscopy quality. However, the programs are significantly variable in terms of methods used to collect quality metrics, specific quality measures obtained, and how quality is reported.
The authors’ work is novel in that this is the first report of the status of colonoscopy quality assurance programs in a large U.S. health care system. The VA health care system is the largest integrated health system in the U.S., serving more than 9 million veterans annually. This survey’s high response rate further strengthens the findings. Specifically, the survey found that VA sites are making a strong concerted effort to measure and report colonoscopy quality. However, there is significant variability in documentation, measurement, and reporting practices. Moreover, the majority of VA sites do not have formal performance improvement plans in place for endoscopists who do not meet thresholds for colonoscopy quality.
[embed:render:related:node:158282]
Screening colonoscopy for CRC offers known mortality benefits to patients.1,17-19 Significant prior work has described and validated the importance of colonoscopy quality metrics, including bowel preparation quality, cecal intubation rate, and ADR and their association with interval colorectal cancer and death.20-23 Gastroenterology professional societies, including the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, have recommended and endorsed measurement and reporting of colonoscopy metrics.24 There is general agreement among endoscopists that colonoscopy quality is an important aspect of performing the procedure.
The lack of formal performance improvement programs is a key finding of this survey. Recent studies have shown that improvements in quality metrics, such as the ADR, by individual endoscopists result in reductions in interval colorectal cancer and death.25 Kahi and colleagues previously showed that providing a quarterly report card improves colonoscopy quality.26 Keswani and colleagues studied a combination of a report card and implementation of standards of practice with resultant improvement in colonoscopy quality.27 Most recently, in a large prospective cohort study of individuals who underwent a screening colonoscopy, 294 of the screening endoscopists received annual feedback and quality benchmark indicators to improve colonoscopy performance.25 The majority of the endoscopists (74.5%) increased their annual ADR category over the study period. Moreover, patients examined by endoscopists who reached or maintained the highest ADR quintile (> 24.6%) had significantly lower risk of interval CRC and death. The lack of formal performance improvement programs across the VHA is concerning but reveals a significant opportunity to improve veteran health outcomes on a large scale.
This study’s findings also highlight the intense resources necessary to measure and report colonoscopy quality. The ability to measure and report quality metrics requires having adequate documentation and data to obtain quality metrics. Administrative databases from electronic health records offer some potential for routine monitoring of quality metrics.28 However, most administrative databases, including the VA Corporate Data Warehouse (CDW), contain administrative billing codes (ICD and CPT) linked to limited patient data, including demographics and structured medical record data. The actual data required for quality reporting of important metrics (bowel preparation quality, cecal intubation rates, and ADRs) are usually found in clinical text notes or endoscopic note documentation and not available as structured data. Due to this issue, the majority of VA sites (79.2%) are using manual chart review to collect quality metric data, resulting in widely variable estimates on time burden. A minority of sites in this study (39.6%) reported using automated endoscopic software reporting capability that can help with the time burden. However, even in the VA, an integrated health system, a wide variety of software brands, documentation practices, and photo documentation was found.
Future endoscopy budget and purchase decisions for the individual VA sites should take into account how new technology and software can more easily facilitate accurate quality reporting. A specific policy recommendation would be for the VA to consider a uniform endoscopic note writer for procedure notes. Pathology data, which is necessary for the calculation of ADR, also should be available as structured data in the CDW to more easily measure colonoscopy quality. Continuous measurement and reporting of quality also requires ongoing information technology infrastructure and quality control of the measurement process.