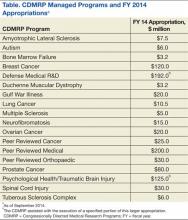
The Congressionally Directed Medical Research Programs (CDMRP), an office within the U.S. Army Medical Research and Materiel Command, has executed funding for research in 18 biomedical programs (Table). These programs touch the lives of service members, veterans, family members, and the general public. A partnership with the military, government, scientific community, survivors, patients, and their family members brings together spheres of stakeholders that typically might not otherwise collaborate and enables the CDMRP to complement other sources of research funding while focusing on research most directly relevant to each disease, condition, or injury.
The CDMRP began in 1992 when the breast cancer advocacy community launched a grassroots effort to raise public awareness of the need for increased federal funding for breast cancer research. These advocates requested from Congress additional research funding to support innovative, high-impact research where the government was willing to take a risk to leapfrog the field forward. In response, Congress added funds to the DoD budget for breast cancer research, and the Breast Cancer Research Program (BCRP) was established with a fiscal year (FY) 1992 congressional appropriation.
The CDMRP brought a flexible, efficient way of managing research and was enthusiastic about the advocates’ desire to have a voice in setting research priorities. Since the initial appropriation, advocates representing breast cancer, ovarian cancer, prostate cancer, neurofibromatosis, and a wide range of other diseases, conditions, and injuries have demonstrated the need to Congress to appropriate funds for their respective causes.
There are several features that differentiate the CDMRP from other funding agencies. The most significant differences follow:
- The CDMRP funds innovative high-risk/high-gain research focused on the disease, condition, or injury as specified in congressional language;
- Unlike other agencies, the CDMRP integrates patients, survivors, family members, or caregivers of a person living with the disease, condition, or injury into every aspect of the program management cycle; and
- Every year the CDMRP programs develop a new investment strategy and release award mechanisms based on the most critical needs and scientific gaps.
These features ensure that the research funded in each program is relevant and has a high potential for impact in the patient community.
Funding and Science Management
Funding for the programs managed by the CDMRP does not appear as part of the DoD core funding in the president’s budget; instead, Congress assesses the needs of its constituents and adds funding to the DoD budget, designated specifically to meet those needs on an annual basis. Management of the CDMRP is funded entirely out of the annual appropriation, and there is no financial burden to the DoD. Unlike other federally funded agencies that receive funding in the president’s budget every FY, each CDMRP program develops an investment strategy based on a single yearly congressional appropriation.
Full project funding is obligated at the start from the single FY appropriation, ensuring multiyear research projects are not at funding risk. This method is in contrast to other agencies, which fund projects in budget years and may fund only a percentage of previously committed levels or cut the length of time for funding, depending on varying budget year funding policies.
Each CDMRP research program is managed by a multidisciplinary team and includes an external advisory board composed of world-renowned expert scientists, clinicians, and survivors from the DoD, National Institutes of Health (NIH), Centers for Disease Control and Prevention, VA, as well as academia and industry. Each research program has a vision/mission that is focused on ending or curing that disease, condition, or injury, ameliorating its consequences, or having a major impact on the quality of life of its survivors. Establishing a vision is the first major milestone in program execution, which enables each program to develop its individual investment strategy.
When establishing the investment strategy, each program evaluates the funding landscape by comparing research portfolios and award mechanisms within the organization as well as with other federal and nonfederal agencies. For some of the CDMRP-managed programs, such as the Peer Reviewed Orthopaedic Research Program, the Spinal Cord Injury Research Program, and the Psychological Health/Traumatic Brain Injury Research Program, topic areas are aligned with the Defense Health Program (DHP) Defense Medical Research and Development Program (DMRDP). The appropriate DHP Joint Program Committee provides guidance on military-relevant research priorities and uses oversight of all core and congressional special interest research efforts across the DoD services to complement and leverage projects with CDMRP funding.
Establishment of each program’s vision and investment strategy leads to the development of Program Announcements (PAs), which describe the intent of each award mechanism in order to solicit research applications aimed at making a significant and nonincremental impact. The PAs for each program as well as links to application submission are made available on the CDMRP webpage (http://cdmrp.army.mil/funding/prgdefault.shtml).