Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
AP is the one of the most common gastrointestinal disorders requiring hospitalization, accounting for roughly 270,000 admissions annually in the U.S., which translates into a $2.6 billion annual health care expenditure.Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
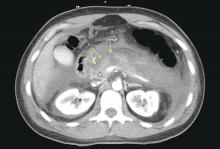
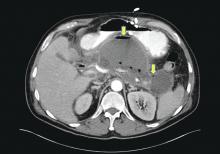
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
As with any disorder, management starts with prevention. Primary prevention of AP has only been well studied in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP). Post-ERCP pancreatitis (PEP) is the most common and arguably the most dreaded complication of ERCP with reported incidence of approximately 10%7. Several medications and endoscopic interventions have been assessed for the prevention of PEP. Of these, placement of prophylactic pancreatic duct stents8,9 and administration of rectal nonsteroidal anti-inflammatory drugs, especially indomethacin, have shown significant benefit in reducing risk for PEP10,11. It is unclear at this point whether rectal indomethacin alone (without pancreatic duct stenting) is sufficient in patients at high risk for PEP. The SVI (Stent Vs. Indomethacin) trial12, an ongoing multicenter randomized controlled trial, aims to answer this specific question.Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
There is a plethora of scoring systems developed to predict AP severity and outcomes at presentation and/or within the first 24 hours. These include the Ranson’s criteria described in 1974, the APACHE-II (Acute Physiology and Chronic Health Evaluation II), BISAP (Bedside Index of Severity in Acute Pancreatitis) scores, and others. They all have similar, but only modest, accuracy16,17. Experts recommend18 that the Systemic Inflammatory Response Syndrome (SIRS) may be the most useful score in daily clinical practice, given that all of its four parameters are readily available (temperature, heart rate, respiratory rate, and white blood cell count) and the score is easy to calculate. Recent studies suggest that admission hematocrit and rise in blood urea nitrogen (BUN) at 24 hours are as accurate as more complex scoring systems in predicting severe disease19.
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
What remains debatable is the amount and type of fluid administered. Lactated Ringers (LR) is likely the optimal solution, based on a small prospective randomized-controlled study showing that administration of LR reduced SIRS compared with saline24. Endpoints to guide adequacy of fluid resuscitation in the first 24-48 hours include measurement of urine output (at least 0.5 mL/kg per hour)25, decrease in hematocrit26 and BUN levels27.