Editor's Note
Gastroenterologists are becoming increasingly involved in the management of obesity. While prior therapy for obesity was mainly based on lifestyle changes, medication, or surgery, the new and exciting field of endoscopic bariatric and metabolic therapies has recently garnered incredible attention and momentum.
In this quarter’s In Focus article, brought to you by The New Gastroenterologist, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital) provide an outstanding overview of the gastric and small bowel endoscopic interventions that are either already approved for use in obesity or currently being studied. This field is moving incredibly fast, and knowledge and understanding of these endoscopic therapies for obesity will undoubtedly be important for our field.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Obesity is a rising pandemic. As of 2016, 93.3 million U.S. adults had obesity, representing 39.8% of our adult population.1 It is estimated that approximately $147 billion is spent annually on caring for patients with obesity. Traditionally, the management of obesity includes lifestyle therapy (diet and exercise), pharmacotherapy (six Food and Drug Administration–approved medications for obesity), and bariatric surgery (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]). Nevertheless, intensive lifestyle intervention and pharmacotherapy are associated with approximately 3.1%-6.6% total weight loss (TWL),2-7 and bariatric surgery is associated with 20%-33.3% TWL.8 However, less than 2% of patients who are eligible for bariatric surgery elect to undergo surgery, leaving a large proportion of patients with obesity untreated or undertreated.9
 Copyright Elsevier and AGA Institute (2017)
Copyright Elsevier and AGA Institute (2017)
Figure 1. Endoscopic bariatric and metabolic therapies (EBMTs): A) Orbera intragastric balloon system, B) ReShape integrated dual balloon system, C) Obalon balloon system, D) Spatz adjustable balloon system, E) Elipse balloon, F) endoscopic sutured/sleeve gastroplasty (ESG), G) primary obesity surgery endoluminal (POSE), H) aspiration therapy, I) transpyloric shuttle, J) duodenal-jejunal bypass liner, K) duodenal mucosal resurfacing, L) gastroduodenojejunal bypass, M) incisionless magnetic anastomosis system. This figure was adapted from an article published in Clinical Gastroenterology and Hepatology 2017;15(5):619-30.
Endoscopic bariatric and metabolic therapies (EBMTs) encompass an emerging field for the treatment of obesity. In general, EBMTs are associated with greater weight loss than are lifestyle intervention and pharmacotherapy, but with a less- invasive risk profile than bariatric surgery. EBMTs may be divided into two general categories – gastric and small bowel interventions (Figure 1 and Table 1). Gastric EBMTs are effective at treating obesity, while small bowel EBMTs are effective at treating metabolic diseases with a variable weight loss profile depending on the device.10,11
Of note, a variety of study designs (including retrospective series, prospective series, and randomized trials with and without shams) have been employed, which can affect outcomes. Therefore, weight loss comparisons among studies are challenging and should be considered in this context.
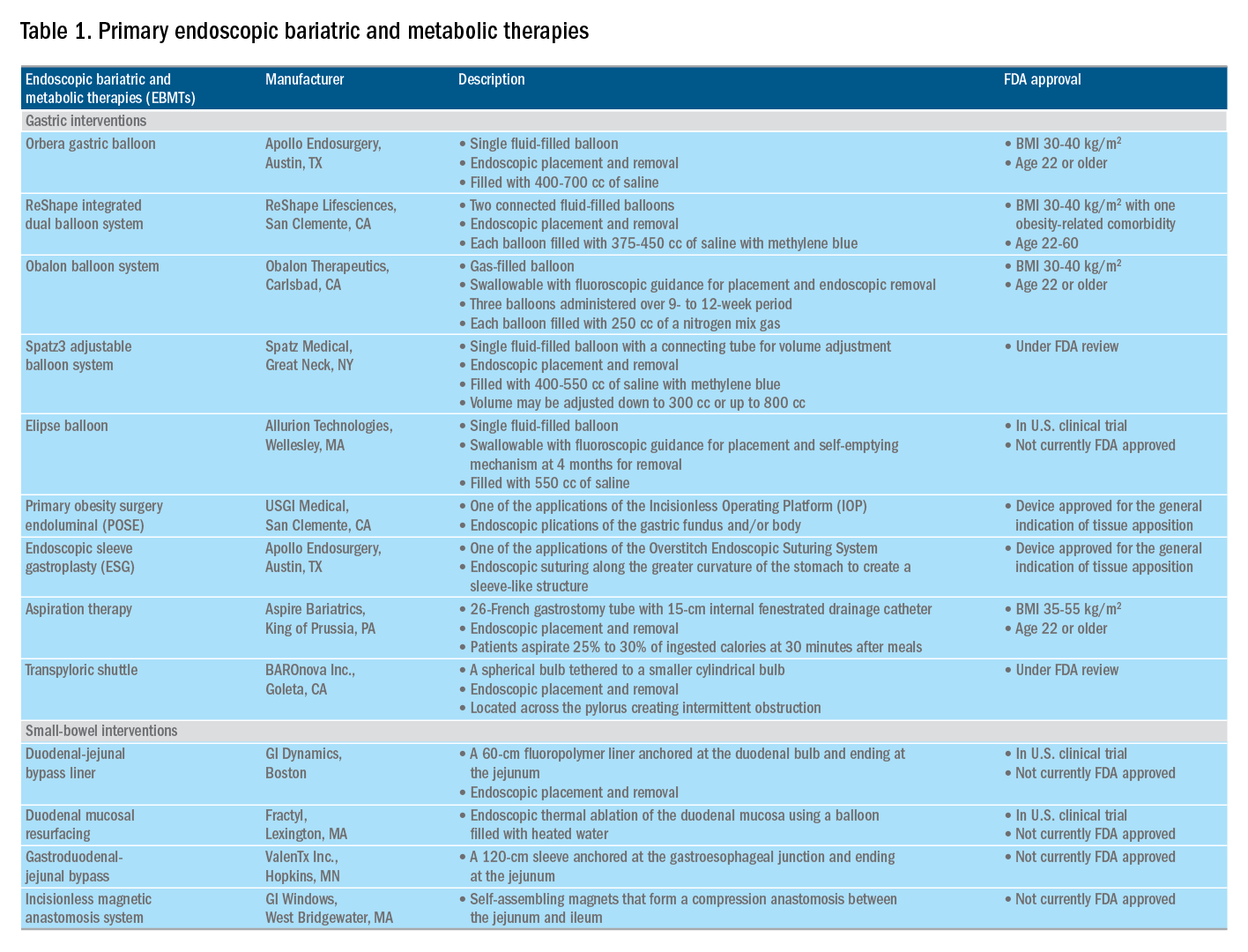
Gastric interventions
Currently, there are three types of EBMTs that are FDA approved and used for the treatment of obesity. These include intragastric balloons (IGBs), plications and suturing, and aspiration therapy (AT). Other technologies that are under investigation also will be briefly covered.
Intragastric balloons
An intragastric balloon is a space-occupying device that is placed in the stomach. The mechanism of action of IGBs involves delaying gastric emptying, which leads to increased satiety.12 There are several types of IGBs available worldwide differing in techniques of placement and removal (endoscopic versus fluoroscopic versus swallowable), materials used to fill the balloon (fluid-filled versus air-filled), and the number of balloons placed (single versus duo versus three-balloon). At the time of this writing, three IGBs are approved by the FDA (Orbera, ReShape, and Obalon), all for patients with body mass indexes of 30-40 kg/m2, and two others are in the process of obtaining FDA approval (Spatz and Elipse).
Orbera gastric balloon (Apollo Endosurgery, Austin, Tex.) is a single fluid-filled IGB that is endoscopically placed and removed at 6 months. The balloon is filled with 400-700 cc of saline with or without methylene blue (to identify leakage or rupture). Recently, Orbera365, which allows the balloon to stay for 12 months instead of 6 months, has become available in Europe; however, it is yet to be approved in the United States. The U.S. pivotal trial (Orbera trial) including 255 subjects (125 Orbera arm versus 130 non-sham control arm) demonstrated 10.2% TWL in the Orbera group compared with 3.3% TWL in the control group at 6 months based on intention-to-treat (ITT) analysis. This difference persisted at 12 months (6 months after explantation) with 7.6% TWL for the Orbera group versus 3.1% TWL for the control group.13,14
ReShape integrated dual balloon system (ReShape Lifesciences, San Clemente, Calif.) consists of two connected fluid-filled balloons that are endoscopically placed and removed at 6 months. Each balloon is filled with 375-450 cc of saline mixed with methylene blue. The U.S. pivotal trial (REDUCE trial) including 326 subjects (187 ReShape arm versus 139 sham arm) demonstrated 6.8% TWL in the ReShape group compared with 3.3% TWL in the sham group at 6 months based on ITT analysis.15,16
Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) is a swallowable, gas-filled balloon system that requires endoscopy only for removal. During placement, a capsule is swallowed under fluoroscopic guidance. The balloon is then inflated with 250 cc of nitrogen mix gas prior to tube detachment. Up to three balloons may be swallowed sequentially at 1-month intervals. At 6 months from the first balloon placement, all balloons are removed endoscopically. The U.S. pivotal trial (SMART trial) including 366 subjects (185 Obalon arm versus 181 sham capsule arm) demonstrated 6.6% TWL in the Obalon group compared with 3.4% TWL in the sham group at 6 months based on ITT analysis.17,18
Two other balloons that are currently under investigation in the United States are the Spatz3 adjustable balloon system (Spatz Medical, Great Neck, N.Y.) and Elipse balloon (Allurion Technologies, Wellesley, Mass.). The Spatz3 is a fluid-filled balloon that is placed and removed endoscopically. It consists of a single balloon and a connecting tube that allows volume adjustment for control of symptoms and possible augmentation of weight loss. The U.S. pivotal trial was recently completed and the data are being reviewed by the FDA. The Elipse is a swallowable fluid-filled balloon that does not require endoscopy for placement or removal. At 4 months, the balloon releases fluid allowing it to empty and pass naturally. The U.S. pivotal trial (ENLIGHTEN trial) is currently underway.
A meta-analysis of randomized controlled trials revealed improvement in most metabolic parameters (diastolic blood pressure, fasting glucose, hemoglobin A1c, and waist circumference) following IGB compared with controls.19 Nausea and vomiting are seen in approximately 30% and should be addressed appropriately. Pooled serious adverse event (SAE) rate was 1.5%, which included migration, perforation, and death. Since 2016, 14 deaths have been reported according to the FDA MAUDE database. Corporate response was that over 295,000 balloons had been distributed worldwide with a mortality rate of less than 0.01%.20