In the 9 months pre-intervention, HIT and HITT occurred in zero of 6717 patients receiving at least 1 dose of VTE prophylaxis. In the 25 months of post-intervention follow-up, 44,240 patients received prophylaxis with either heparin. HIT (clinical suspicion with positive antibody and confirmatory SRA) occurred in 3 patients, 2 of whom had HITT, all after UFH. This incidence was not statistically significant using chi square analysis ( P = 0.86).
Discussion
Because the efficacy of UFH and LMWH for VTE prophylaxis are equivalent [6],choosing between them involves many factors including patient-level risk factors such as renal function, risk of bleeding, as well as other considerations such as nursing time, patient preference, risk of HIT, and acquisition cost. Indeed, the most recent version of the American College of Chest Physicians guidelines for prophylaxis against VTE note that both drugs are recommended with an evidence grade of IB [7].Cost is among the considerations considered appropriate in choosing among agents. The difference in acquisition costs of > $20 per patient per day can have a major financial impact on hospital’s pharmacy budget and may be decisive. But a focus only on acquisition cost is short sighted as the 2 medications have different complication rates with regard to HIT. Thus the need to track HIT incidence after protocol changes are made is paramount.
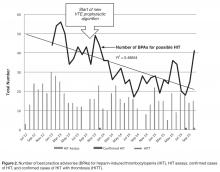
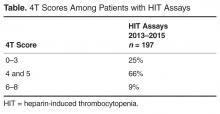
In our study, we did not measure thrombocytopenia as an endpoint because acquired thrombocytopenia is too common and multifactorial to be a meaningful. Rather, we used the clinical suspicion for HIT as measured by both the number of times the BPA fired warnings of low platelets in the setting of recent heparin use and the number of times clinicians suspected HIT enough to order a HIT assay. We also used actual outcomes (clinically adjudicated cases of HIT and HITT). Our data shows substantial compliance among clinicians with the voluntary conversion to UFH with an immediate and sustained shift to UFH so that UFH was used in 86% of patients. Corresponding cost savings were achieved in heparin acquisition. Unlike some prior reports, there was a minimal burden of HIT as measured by the unchanged number of BPAs, monthly HIT assays and the unchanged clinical risk 4T scores among those patients in whom the test was ordered pre and post intervention. HIT rates were not statistically different after the order set conversion took effect.