Results
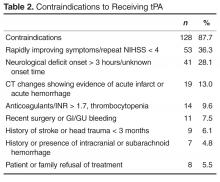
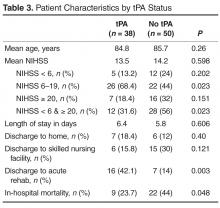
From 1 January 2011 to 30 November 2013, a total of 984 people presented to the ED with acute neurological changes concerning for ischemic stroke. Of those, 184 people (18.7%) were 80 years or older with an average age of 85.3 (range, 80–96). Patient characteristics are presented in Table 1 . The average NIHSS was 12 (range, 1–32). Thirty-four (18.5%) patients presented with severe stroke symptoms (NIHSS ≥ 20), while moderate symptoms (NIHSS 6–19) and mild symptoms (NIHSS < 6) accounted for 97 (52.7%) and 52 (28%) cases, respectively. Age and presenting NIHSS were positively correlated ( P = 0.002). The overall in-hospital mortality rate for the population was 23.4%. Those with presenting NIHSS > 20 were more likely to experience in-hospital death ( P < 0.001). Thirty-eight patients (20.7%) received tPA and had an average age of 84.8 years, while 146 (79.3%) did not receive tPA and had an average age of 85.4 years. Of those that did not receive tPA, 128 (87.7%) had 1 or more clearly documented contraindications ( Table 2 ). Ten patients (6.8%) were excluded due to clinical concerns including comorbidities, debility, or advanced dementia. Fifty-three (36.3%) of patients had rapidly improving stroke symptoms with repeat NIHSS < 4. Of those with contraindications, 49 (33.6%) had arrival outside the 3-hour treatment window, unknown time of onset, or developing radiographic changes on CT representing the natural history of stroke progression. Fourteen (9.6%) were on anticoagulants including warfarin and dabigatran with elevated INR or thrombocytopenia. Seven (4.8%) had a history of intracranial hemorrhages and 11 (7.5%) had recent surgery or bleeding episodes. One patient was not treated due to hospice enrollment. Only 8 (5.5%) patients declined treatment with tPA. Those with contraindications including rapidly improving symptoms, treatment with anticoagulants with therapeutic indices, recent bleeding episodes, or family refusal were excluded from comparative analyses. The remaining 50 patients were included in comparative analysis ( Table 3 ). There was no difference between the tPA and non-tPA groups in age ( P = 0.26). While overall there was no difference between groups in initial NIHSS ( P = 0.598), more patients with moderate symptoms (NIHSS 6-19) received tPA ( P = 0.023). Similarly, those who did not receive tPA were more likely to have presented with mild or severe symptoms ( P = 0.023). There was no significant difference in length of stay between the tPA group (6.4 days) and non-tPA group (5.8 days) ( P =0.606). Sixteen (42.1%) patients who received tPA were discharged to acute rehabilitation hospitals, compared to 7 (14%) of those that did not receive tPA ( P =0.003). There was no difference between groups in the numbers discharged to home ( P = 0.40) or to skilled nursing facilities ( P = 0.121). Those who receive tPA were less likely to experience in hospital death ( P = 0.048). Only 1 patient (2.6%) who received tPA, versus zero who did not receive tPA, developed symptomatic ICH ( P = 0.249).
Discussion
Ischemic stroke remains a major cause of morbidity and mortality for very old patients. Though less than 5% of the United States population is over the age of 80 [1], at this community-based hospital 18% of those presenting to the ED with ischemic stroke were in this age-group. With a population of increasing age, more people in this age-group will present with ischemic stroke and need effective treatment to limit the associated morbidity and mortality. Being able to quickly and safely treat acute ischemic stroke may help very old adults maintain independence or prevent institutionalization. While the original studies demonstrating the effectiveness of tPA for acute ischemic stroke excluded or underrepresented those ≥ 80 years, retrospective analysis has not been conclusive regarding its use in very old patients [4–6,10,12,13].However, post-hoc analysis of NINDS and IST-3 data demonstrate efficacy and safety of treatment [12,13].