From the University of Connecticut School of Medicine, Farmington, CT.
Abstract
- Objective: To review the metabolic complications of HIV infection.
- Methods: Review of the literature in the context of 3 clinical cases.
- Results: People with HIV infection are living longer thanks to the advent of potent antiretroviral therapy. This has led to increased incidence of age-related metabolic complications, including a higher risk of cardiovascular disease, hyperlipidemia, metabolic syndrome, and osteoporosis. Appropriate management of these complications requires an understanding of disease-related and drug-related side effects as well as the potential for drug-drug interactions. A multidisciplinary approach to patient management is most effective.
- Conclusion: Awareness of the metabolic complications frequently encountered in HIV infection, drug interactions, and possible toxicities is critical to the successful management of HIV-infected individuals.
Key words: HIV; antiretroviral therapy; hyperlipidemia; metabolic syndrome; diabetes; hypogonadism.
According to the most recent data from the Joint United Nations Programme on HIV/AIDS (UNAIDS), 36 million people worldwide are living with HIV/AIDS, with 18 million accessing effective antiretroviral therapy (ART) [1]. The past 2 decades have witnessed enormous advances in the field from prevention to diagnosis and therapeutics, and modern ART largely allows HIV-infected persons to live near-normal life spans [2,3]. However, from the beginning of the epidemic, HIV-infected persons on effective therapy have suffered from myriad metabolic consequences, many of which affect quality of life and result in excess mortality [4]. It is also true that untreated HIV infection portends an increased risk of metabolic complications, likely related to abnormal immune activation, as demonstrated in structured interruption trials [5,6]. It is worth noting, however, that while many of these metabolic dyscrasias and associated risks have historically been attributed primarily to the treatment of HIV infection with ART, data from cohort studies and randomized clinical trials have repeatedly demonstrated significant reductions in morbidity and mortality when ART is initiated early [7]. In this paper, we review HIV-related metabolic complications frequently encountered in clinical practice (hyperlipidemia, diabetes, and bone disease) and best practice considerations in the context of 3 clinical cases.
Case Patient 1
Initial Presentation and History
A 58-year-old male with a history of hypertension and mixed hyperlipidemia is referred for evaluation of newly diagnosed HIV infection. He has no history of intravenous drug use but has had multiple male and female sex partners in the past few years, and requested testing after a partner tested positive. His last negative test was 2 years ago. The patient does not smoke cigarettes. Overall he feels well and tolerates his regimen of lisinopril 10 mg and simvastatin 20 mg daily. On initial evaluation, his exam is unremarkable other than subtle white plaques on the dorsal surface of the tongue and buccal mucosa, and moderate central obesity. Vital signs including blood pressure are normal. Initial laboratory evaluation reveals a CD4 cell count of 150 cells/mm3 and an HIV RNA level of 200,000 copies/mL. Fasting serum total cholesterol is 220 mg/dL, triglycerides 250 mg/dL, LDL 170 mg/dL, and HDL 35 mg/dL. Serum BUN, creatinine, and liver function testing results are normal.
What initial regimen might be recommended based on the status of his HIV infection and comorbidities?
The most recent iteration of the US Department of Health and Human Services (DHHS) guidelines on use of antiretroviral agents (ARVs) in HIV recommends an initial ART regimen that includes a backbone of 2 nucleoside reverse transcriptase inhibitors (NRTIs), generally tenofovir disoproxil fumarate or tenofovir alafenamide, abacavir (ABC), emtricitabine (FTC), or lamivudine (3TC) [2]. To this backbone should be added a third agent; the majority of those currently recommended are integrase strand transfer inhibitors (INSTIs) (dolutegravir, elvitegravir, raltegravir); one recommended protease inhibitor (PI) (ritonavir-boosted darunavir) is also an option. Some of these initial recommended regimens are available as fixed-dosed combinations in 1 pill, making them attractive options.
The latest guidelines also clearly recommend starting ART in all HIV-infected individuals, irrespective of CD4 count. The patient described above has a very low CD4 count, so there is no question he needs to begin therapy promptly. Given his low CD4 count and relatively high viral load, one may consider a ritonavir-boosted PI as perhaps the most robust option and with a relatively high barrier to resistance development, in contrast to other options. Assuming the patient’s baseline resistance testing reveals a fully sensitive wild-type virus without meaningful resistance mutations, he will begin a regimen of TDF/FTC plus ritonavir-boosted darunavir, 3 pills once daily. Given his low CD4 count (below 200 cells/mm3), he will also need prophylaxis for Pneumocystis jirovecii pneumonia, in the form of trimethoprim/sulfamethoxazole (TMP/SMX) daily. Given the potential for interaction between the boosted PI and simvastatin, his lipid-lowering agent is switched to atorvastatin 10 mg daily.
What is the association between hyperlipidemia and HIV infection and treatment?
Hyperlipidemia represents a key modifiable risk factor for the development of cardiovascular disease (CVD) in HIV-infected individuals [8]. Indeed, a multicenter cross-sectional study of older HIV-infected individuals performed in Spain revealed a 54% prevalence of dyslipidemia and 23% CVD [9]. Most experts believe that metabolic abnormalities observed in HIV-infected individuals are the result of a combination of factors: those resulting from abnormal immune activation and inflammation related to viral replication, and those related to certain ARVs [10].
Early after HIV seroconversion, decline in HDL is one of the first proatherogenic changes observed. This, along with increased triglyceride and LDL levels, likely contribute to increased risk of CVD in this population. Increased microbial translocation, evidenced by increased levels of lipopolysaccharide (LPS), may drive immune activation, leading to dyslipidemia via a multitude of hypothesized mechanisms [4]. It has been theorized that HDL lipoproteins are less stable on ART, leading to potentially impaired plasma lipolytic activities or hepatic cholesteryl ester uptake [6,11]. Increased VLDL from release of free fatty acids may lead to higher triglyceride levels and triglyceride-rich LDL and HDL, all associated with increased risk of CVD [11].
In terms of effects of specific ARV classes, although newer agents have less of a propensity to cause dyslipidemia, the PI class arguably remains most problematic. In comparison to other classes, the PIs tend to result in greater increases in triglycerides, total cholesterol, and LDL, and have frequent drug-drug interactions with lipid-lowering agents [10,12]. Estimated prevalence of dyslipidemia in patients receiving PI therapy varies from 28% to 80% [13]. The prospective multinational cohort Data collection on Adverse events of Anti-HIV Drugs (DAD) study found significantly higher rates of hypertriglyceridemia, hypercholesterolemia, and low HDL in patients on PIs in comparison to non–nucleoside reverse transcriptase inhibitors (NNRTIs) [14]. Various mechanisms have been proposed to explain the PIs adverse effects on lipids, including inhibition of lipogenesis and adipocyte differentiation, decreased hepatocyte clearance of chylomicrons and VLDL, and increased hepatic synthesis of triglycerides [15]. Of the available PIs, atazanavir and darunavir have less potential to lead to dyslipidemia [10], while lopinavir/ritonavir, fosamprenavir, and tipranavir may have the highest [13]. Of the NNRTIs, efavirenz is most frequently associated with dyslipidemia, specifically increased triglycerides and total cholesterol [13]. However, these increased values seen on efavirenz therapy may be offset by relative increases in HDL, with little resultant effect on the total cholesterol:HDL ratio. Rilpivirine, etravirine, and nevirapine are relatively less likely to drive lipid changes, although certain drug interactions are important to recognize in clinical practice, such as the interaction between rilpivirine and proton pump inhibitors [2,13,16]. It is also worth noting that no NNRTIs are included in current guidelines as preferred therapy [2].
Historically, the thymidine analogue NRTIs (stavudine, didanosine, zidovudine) have been associated with lipid dyscrasias and lipoatrophy, but fortunately these are no longer used frequenty except in cases requiring deep salvage therapy for highly treatment-experienced patients. Two newer NRTIs, tenofovir and abacavir, have relatively neutral to favorable effects on lipids. The combination of tenofovir disoproxil (TDF) and emtricitabine (trade name Truvada) was associated with significantly lower triglycerides, total cholesterol and LDL than other NRTI pairs [6]. TDF has been postulated to have lipid-lowering effects. Switch studies in which patients were taken off thymidine analogues and placed on TDF, demonstrated recovery of limb fat in patients with lipoatrophy, and those switched off abacavir-based ART to TDF showed statistically significant lower fasting total cholesterol at week 12, without differences of viral suppression [8]. Tenofovir alafenamide (TAF) is a next-generation prodrug of tenofovir that results in improved stability in plasma and higher intracellular levels in comparison to TDF [17]. Although randomized controlled trials of TAF vs TDF-based ARV regimens have suggested statistically higher total cholesterol, serum HDL is also increased resulting in unchanged total:HDL ratios and no differences in risk classifications [18]. Integrase inhibitors (INSTI) now represent first-line therapy in combination with an NRTI backbone, and since their availability in 2007 have been evaluated in comparison to various PIs and NNRTIs. Both raltegravir and dolutegravir have consistently shown broad neutral effects on lipids and are among the most metabolically friendly agents available [19–21]. Because it is given in fixed-dose combination with non-ritonavir pharmacologic booster cobicistat, elvitegravir has effects similar to ritonavir-boosted PIs on lipids [6].
What are management considerations in the treatment of hyperlipidemia in HIV-infected patients?
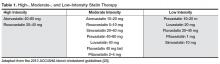
Patients with HIV and hyperlipidemia may benefit from lipid-lowering therapy in addition to ART, although in certain cases appropriate switches may make a difference. Careful consideration of drug interactions between ARVs and lipid-lowering agents, in addition to ARV history and known drug resistance, is warranted prior to selecting a regimen in these patients. In addition, the latest American College of Cardiology/American Heart Association guidelines suggest evaluating 10-year risk of atherosclerotic cardiovascular disease (ASCVD) using the pooled cohort equation to determine the type and dose of statin required (moderate vs high intensity) [22]. It is noteworthy that HIV infection and its therapies are not taken into account as potential risk factors in this model. Primary prevention in non-diabetic patients with a statin is recommended for patients with a 10-year absolute risk of ≥ 7.5% [22]. This patient’s risk is estimated at between 12% and 13% based on this equation, so primary prevention with a moderate-or high-intensity statin is recommended (Table 1) [23]. Data from more than 80,000 patients in the Veterans Aging Cohort Study (VACS) showed that HIV-infected patients with no baseline ASCVD had 50% increased risk of acute myocardial infarction when compared to HIV-uninfected patients over 6 years of follow-up [24]. Thus, consideration of the virus itself or its therapy as an additional risk factor may be valid.