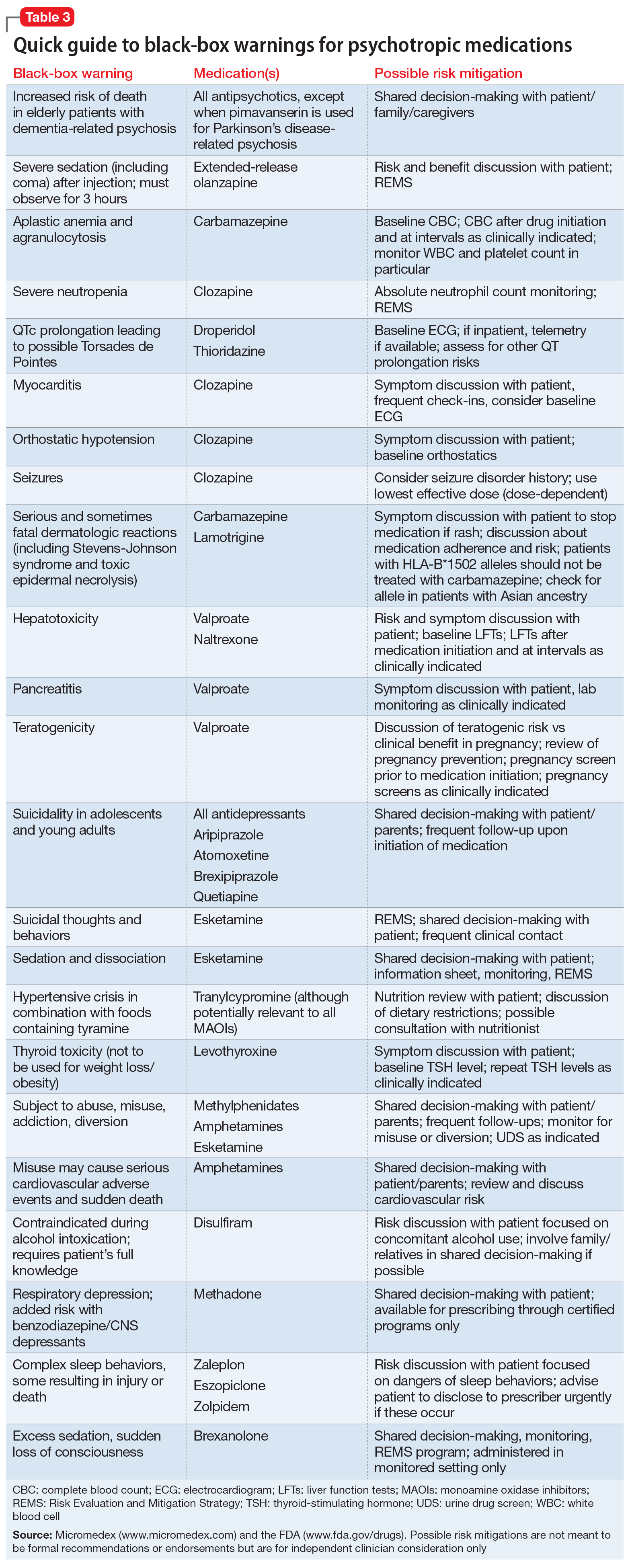
Psychotropic medications and black-box warnings
Psychotropic medications have a large number of black-box warnings.14 Because it is difficult to find black-box warnings for multiple medications in one place, we have provided 2 convenient resources to address this gap: a concise summary guide (Table 3) and a more detailed database (Table 4, Table 5, Table 6, Table 7, and Table 8). In these Tables, the possible risk mitigations, off-label uses, and monitoring are not meant to be formal recommendations or endorsements but are for independent clinician consideration only.
The information in these Tables was drawn from publicly available data, primarily the Micromedex and FDA web sites (see Related Resources). Because this information changes over time, at the end of this article we suggest ways for clinicians to stay updated with black-box warnings and build on the information provided in this article. These tools can be useful for day-to-day clinical practice in addition to studying for professional examinations. The following are selected high-profile black-box warnings.
Antidepressants and suicide risk. As a class, antidepressants carry a black-box warning on suicide risk in patients age ≤24. Initially issued in 2005, this warning was extended in 2007 to indicate that depression itself is associated with an increased risk of suicide. This black-box warning is used for an entire class of medications as well as for a specific patient population (age ≤24). Moreover, it indicates that suicide rates in patients age >65 were lower among patients using antidepressants.
Among psychotropic medication black-box warnings, this warning has perhaps been the most controversial. For example, it has been suggested that this black-box warning may have inadvertently increased suicide rates by discouraging clinicians from prescribing antidepressants,15 although this also has been called into question.16 This black-box warning illustrates that the consequences of issuing black-box warnings can be very difficult to assess, which makes their clinical effects highly complex and challenging to evaluate.14
Antipsychotics and dementia-related psychosis. This warning was initially issued in 2005 for second-generation antipsychotics and extended to first-generation antipsychotics in 2008. Antipsychotics as a class carry a black-box warning for increased risk of death in patients with dementia (major neurocognitive disorder). This warning extends to the recently approved antipsychotic pimavanserin, even though this agent’s proposed mechanism of action differs from that of other antipsychotics.17 However, it specifically allows for use in Parkinson’s disease psychosis, which is pimavanserin’s indication.18 In light of recent research suggesting pimavanserin is effective in dementia-related psychosis,19 it bears watching whether this agent becomes the first antipsychotic to have this warning removed.
Continue to: This class warning has...