Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
[embed:render:related:node:108945]
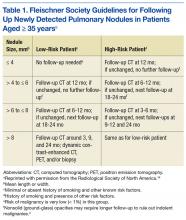
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly selected for noncontrasted CT image review and data collection. Each CT image was de-identified and then reviewed by a pulmonologist and a radiologist. The study used 4 reviewers—2 board-certified pulmonologists and 2 board-certified radiologists—all with > 3 years of experience. Both radiologists were thoracic specialists, and no residents or fellows participated. For each case, reviewers were given a brief patient history outlining smoking and other malignancies. Data collected included age, sex, race, exposure to cigarette smoking, and the gold standard of final diagnosis (FD).
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).