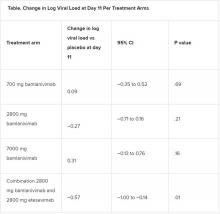
A combination treatment of neutralizing monoclonal antibodies bamlanivimab and etesevimab was associated with a statistically significant reduction in SARS-CoV-2 at day 11 compared with placebo among nonhospitalized patients who had mild to moderate COVID-19, new data indicate.
However, bamlanivimab alone in three different single-infusion doses showed no significant reduction in viral load, compared with placebo, according to the phase 2/3 study by Robert L. Gottlieb, MD, PhD, of the Baylor University Medical Center and the Baylor Scott & White Research Institute, both in Dallas, and colleagues.
Findings from the Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) study were published online Jan. 21 in JAMA. The results represent findings through Oct. 6, 2020.
BLAZE-1 was funded by Eli Lilly, which makes both of the antispike neutralizing antibodies. The trial was conducted at 49 U.S. centers and included 613 outpatients who tested positive for SARS-CoV-2 and had one or more mild to moderate symptoms.
Patients were randomized to one of five groups (four treatment groups and a placebo control), and researchers analyzed between-group differences.
All four treatment arms suggest a trend toward reduction in viral load, which was the primary endpoint of the trial, but only the combination showed a statistically significant reduction.
The average age of patients was 44.7 years, 54.6% were female, 42.5% were Hispanic, and 67.1% had at least one risk factor for severe COVID-19 (aged ≥55 years, body mass index of at least 30, or relevant comorbidity such as hypertension).
Among secondary outcomes, there were no consistent differences between the monotherapy groups or the combination group versus placebo for the other measures of viral load or clinical symptom scores.
The proportion of patients who had COVID-19–related hospitalizations or ED visits was 5.8% (nine events) for placebo; 1.0% (one event) for the 700-mg group; 1.9% (two events) for 2,800 mg; 2.0% (two events) for 7,000 mg; and 0.9% (one event) for combination treatment.
“Combining these two neutralizing monoclonal antibodies in clinical use may enhance viral load reduction and decrease treatment-emergent resistant variants,” the authors concluded.
Safety profile comparison
As for adverse events, immediate hypersensitivity reactions were reported in nine patients (six bamlanivimab, two combination treatment, and one placebo). No deaths occurred during the study.
Serious adverse events unrelated to SARS-CoV-2 infection or considered related to the study drug occurred in 0% (0/309) of patients in the bamlanivimab monotherapy groups; in 0.9% (1/112) of patients in the combination group; and in 0.6% (1/156) of patients in the placebo group.
The serious adverse event in the combination group was a urinary tract infection deemed unrelated to the study drug, the authors wrote.
The two most frequently reported side effects were nausea (3.0% for the 700-mg group; 3.7% for the 2,800-mg group; 5.0% for the 7,000-mg group; 3.6% for the combination group; and 3.8% for the placebo group) and diarrhea (1.0%, 1.9%, 5.9%, 0.9%, and 4.5%, respectively).
The authors included in the study’s limitations that the primary endpoint at day 11 may have been too late to best detect treatment effects.
“All patients, including those who received placebo, demonstrated substantial viral reduction by day 11,” they noted. “An earlier time point like day 3 or day 7 could possibly have been more appropriate to measure viral load.”
Currently, only remdesivir has been approved by the Food and Drug Administration for treating COVID-19, but convalescent plasma and neutralizing monoclonal antibodies have been granted emergency-use authorization.
In an accompanying editor’s note, Preeti N. Malani, MD, with the division of infectious diseases at the University of Michigan, Ann Arbor, and associate editor of JAMA, and Robert M. Golub, MD, deputy editor of JAMA, pointed out that these results differ from an earlier interim analysis of BLAZE-1 data.
A previous publication by Peter Chen, MD, with the department of medicine at Cedars Sinai Medical Center, Los Angeles, compared the three monotherapy groups (no combination group) with placebo, and in that study the 2,800-mg dose of bamlanivimab versus placebo achieved statistical significance for reduction in viral load from baseline at day 11, whereas the other two doses did not.
The editors explain that, in the study by Dr. Chen, “Follow-up for the placebo group was incomplete at the time of the database lock on Sept. 5, 2020. In the final analysis reported in the current article, the database was locked on Oct. 6, 2020, and the longer follow-up for the placebo group, which is now complete, resulted in changes in the primary outcome among that group.”
They concluded: “The comparison of the monotherapy groups against the final results for the placebo group led to changes in the effect sizes,” and the statistical significance of the 2,800-mg group was erased.
The editors pointed out that monoclonal antibodies are likely to benefit certain patients but definitive answers regarding which patients will benefit and under what circumstances will likely take more time than clinicians have to make decisions on treatment.
Meanwhile, as this news organization reported, the United States has spent $375 million on bamlanivimab and $450 million on Regeneron’s monoclonal antibody cocktail of casirivimab plus imdevimab, with the promise to spend billions more.
However, 80% of the 660,000 doses delivered by the two companies are still sitting on shelves, federal officials said in a press briefing last week, because of doubts about efficacy, lack of resources for infusion centers, and questions on reimbursement.
“While the world waits for widespread administration of effective vaccines and additional data on treatments, local efforts should work to improve testing access and turnaround time and reduce logistical barriers to ensure that monoclonal therapies can be provided to patients who are most likely to benefit,” Dr. Malani and Dr. Golub wrote.
This trial was sponsored and funded by Eli Lilly. Dr. Gottlieb disclosed personal fees and nonfinancial support (medication for another trial) from Gilead Sciences and serving on an advisory board for Sentinel. Several coauthors have financial ties to Eli Lilly. Dr. Malani reported serving on the National Institute of Allergy and Infectious Diseases COVID-19 Preventive Monoclonal Antibody data and safety monitoring board but was not compensated. Dr. Golub disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.