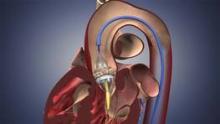
The Food and Drug Administration has expanded approval for the Sapien transcatheter heart valve for patients with aortic valve stenosis who are at high risk for serious surgical complications or death.
It is the second approval for the device, which is made by Edwards Lifesciences Corp. of Irvine, Calif. In November 2011, the FDA approved it for severe aortic valve stenosis in inoperable patients.
The indication for high-risk patients was reviewed by an FDA advisory panel in July. The majority of the panel indicated that the device was safe and effective in that group.
Both approvals were based on results from the PARTNER trial (N. Engl. J. Med. 2010;363:1597-607).
"Any procedure to replace the aortic valve carries the risk for serious complications, but for some patients with coexisting conditions or diseases, that risk may be especially high," Christy Foreman, director of the office of device evaluation at FDA’s Center for Devices and Radiological Health, said in a statement.
"The THV serves as an alternative for some very high-risk patients."
Larry L. Wood, Edwards’ corporate vice president, transcatheter heart valves, said in a statement: "It is extremely rewarding for us and our clinical partners to know that high-risk patients suffering from this often-debilitating disease will now have access to this life-saving therapy."
The Sapien THV is implanted at the site of the diseased valve using a catheter that is delivered either through a transfemoral or transapical approach. According to the Edwards statement, the transapical approach, which requires insertion through the ribs and myocardium, was not approved outside of clinical trials until now.
Transcatheter aortic valve replacement has become one of the driving forces in the heart valve market, which may hit $1.5 billion by 2016.
The new indication for the Sapien THV is expected to expand that market further. According to the FDA, the approved labeling indicates that a surgeon must determine whether a patient is eligible for the Sapien. But in fact, Edwards, the Centers for Medicare and Medicaid Services, and professional societies such as the American College of Cardiology and the Society of Thoracic Surgeons, have worked together to establish standards and requirements for "Heart Teams" that must be present during the procedure and evaluation of the patient by two cardiac surgeons.
There is an increased risk for major vascular complications and for stroke during the first month post implant. Thus, the device is contraindicated in patients who cannot tolerate anticoagulation/antiplatelet therapy.
Edwards will monitor safety and complication rates through the national Transcatheter Valve Therapy Registry.