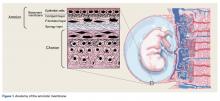
The amniotic membrane is a multilayer tissue forming the innermost layer of the amniotic sac that surrounds the developing fetus. It is comprised of 5 layers, from the inside out: a single layer of epithelial cells, a thick basement membrane, a compact layer, a fibroblast layer, and a spongy layer that abuts the surrounding chorion (Figure 1).1
The amniotic membrane serves several functions, including synthesis of growth factors and cytokines, regulation of pH, transport of water and solutes, and provision of a permeable barrier to amniotic macromolecules.2Amniotic epithelial cells are derived from the pluripotent epiblast at approximately day 8 of gestation. This is well before gastrulation occurs at days 15 to 17, considered the “tipping point” when pluripotent cells differentiate into ectoderm, mesoderm, and endoderm.3 These cells express Oct-4 and Nanog, 2 molecular markers that are indicative of pluripotency.3 Two cell types have been identified in amniotic tissues that possess stem cell-like characteristics: human amniotic epithelial cells and human amniotic mesenchymal stromal cells.4 Both of these cell types have demonstrated the ability to differentiate into various cell lineages, including endothelial cells, adipocytes, myogenic cells, neurogenic cells, chondrocytes, tenocytes, and osteogenic cells.5-7 These previously reported findings indicate that amniotic cells and tissue have the capability to generate mesenchymal tissues.
FDA Classification and Available Forms
The US Food and Drug Administration (FDA) classifies amnion as an allograft tissue under Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 361. To meet criteria, the tissue needs to be minimally manipulated. It is to be for homologous use and cannot be combined with other cells or tissues. There can be no systemic effect or dependence on the metabolic activity of living cells to achieve its primary function. The tissue has to have a localized effect in vivo. Therefore, amnion allograft tissue can be commercialized, provided it is not marketed as a stem cell product or to contain viable cells.
Amniotic tissue is commercially available in several forms.
These include fresh-frozen injectable amniotic liquid that may contain viable amniotic cells and/or particulated amniotic membrane, a micronized freeze-dried (lyophilized) particulate powder that is directly applied to a wound or resuspended for injection, and a cross-linked dehydrated membrane acting as an adhesion barrier (Figure 2).Safety
Amniotic tissue has been used for over 100 years in burn, ophthalmology, and chronic wound patients with favorable outcomes and no adverse effects reported in the literature. Unlike embryonic stem cells, which may be tumorigenic,8 amniotic cells do not possess any known tumorigenicity.9 In one study, 50 immunodeficient mice were injected with 1 to 2 million amniotic epithelial cells and observed for a maximum of 516 days with no tumorigenicity observed in any of the animals.10 In another study, amniotic epithelial cells were implanted into the forearms of healthy volunteers and no immunologic response was observed in any of the recipients.11 Furthermore, viable amniotic cells were recovered via biopsy 7 weeks following transplantation, demonstrating viability of the transplanted cells.11 The lack of tumorigenicity and immunologic response in hosts is due in part to the fact that amniotic cells do not express human leukocyte antigen class II antigens and only express class I antigens in small amounts.3
Advantages of Amnion Tissue
Amniotic tissue is readily available, as it is often discarded after childbirth. The use of this tissue poses no added risk to the fetus or mother, eliminating the ethical concerns associated with obtaining embryonic stem cells. Amniotic tissue is comprised of an extracellular matrix, which acts as a natural scaffold for cellular attachment and structural support for cells as well as collagen types I, III, IV, V, and VI, hyaluronic acid, and a host of growth factors.12 In addition, it possesses antimicrobial properties, including beta-defensins.13
Amniotic tissue has been shown to exert an anti-inflammatory effect by inhibiting the inflammatory cascade. Specifically, it has been shown to inhibit cytokines such as tumor necrosis factor-alpha in the presence of dendritic cells,14 as well as inhibiting transforming growth factor-beta, interleukin-8, and fibroblast proliferation.15 These findings indicate that amniotic tissue has the ability to dampen the “cytokine storm” that occurs after an injury in an adult, which would lead to beneficial impacts on healing and scar formation in patients.16
Basic Science and Animal Studies
Several studies have demonstrated promising outcomes for orthopedic applications in vitro. A comparison of osteogenic potential found that amniotic fluid-derived cells were able to produce approximately 5 times more mineralized matrix than bone marrow-derived mesenchymal stem cells.17 More recently, Si and colleagues18 compared the osteogenic potential of human amniotic epithelial cells, amniotic cells, and human bone marrow-derived mesenchymal stem cells. They found that all 3 cell lines were osteogenic, though the amniotic epithelial cells had better immunomodulatory properties and marginally less osteogenic potential than the other 2 cell types. Furthermore, several in vivo animal studies have demonstrated the ability of human amniotic cells to stimulate bone growth in rats,19,20 rabbits,21 and sheep.22