INTRODUCTION
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading nonobstetric cause of maternal death in the United States and in developed countries. 1,2 During pregnancy, the risk for VTE increases four- to six-fold, and although the risk is present throughout pregnancy, the mother is at highest risk immediately postpartum. 3–5
VTE risk is increased due to physiologic and anatomic changes that occur in pregnancy. These changes include hypercoagulability, progesterone-induced venous stasis, decreased venous outflow, compression of the inferior vena cava and pelvic veins by the expanding uterus, and decreased mobility. The hypercoagulability of pregnancy is due to increased levels of coagulation factors I (fibrinogen), VII, VIII, and X, and von Willebrand factor; decreased free protein S, a natural anticoagulant; acquired resistance to activated protein C; and decreased fibrinolysis due to increased levels of plasminogen activator inhibitor-1 and -2. 6,7 These changes confer increased hemostasis to the mother for delivery but also place her at higher risk for thrombosis.
A review of the literature found that more than 70% of pregnancy-associated DVTs are located in the ileofemoral region, as compared with approximately 9% in non-pregnant patients. 8 The proximal location is associated with a higher risk for post-thrombotic syndrome and embolization as compared with calf DVTs. 9 Proximal postnatal thrombosis, smoking, and older age are independent predictors of the development of post-thrombotic syndrome. 10
RISK FACTORS
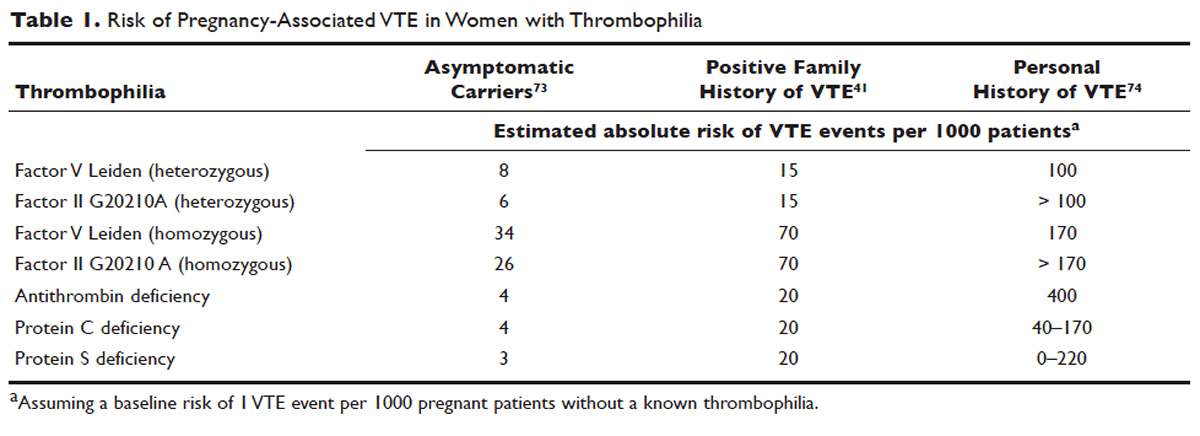
Clinical risk factors that increase the risk for VTE during pregnancy include a prior history of estrogen-related or unprovoked VTE, being a carrier of severe inherited thrombophilia (homozygotes for factor V Leiden or factor II G20210A variants, double heterozygotes, or persons with antithrombin, protein C, or protein S deficiencies), and the presence of antiphospholipid (aPL) antibodies. 11 Women with systemic lupus erythematosus, diabetes, sickle cell disease, and heart disease also have a high risk for VTE during pregnancy. 12 Other risk factors predisposing to thrombosis include black ethnicity, smoking, operative procedures, conception after assisted reproductive techniques, high body mass index, antepartum immobilization, severe preeclampsia, advanced age and parity, and a family history of VTE. 13 A prospective cohort study of 1,297,037 pregnancies and related puerperium identified the following risk factors for thrombosis: hospitalization, infection, hyperemesis, multiple pregnancies, preeclampsia, obesity, cesarean section, major postpartum hemorrhage, intrauterine growth restriction, and fetal death. 14 Risk factors identified in an Agency for Healthcare Research and Quality study include: age 35 or older, black ethnicity, lupus, sickle cell disease, heart disease, postpartum infection, and transfusion. 15 The combination of more than one risk factor increases the risk for VTE. All these factors have to be considered when deciding on prophylactic or therapeutic anticoagulation therapy in pregnancy. In addition, the risks of anticoagulation, including bruising, bleeding, and other side effects (eg, reduced bone mineral density with therapeutic-dose unfractionated heparin), allergic reactions, and rarely thrombocytopenia, must be considered.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION I
A 31-year-old woman G1P0 at 10 weeks’ gestation with no personal or family history of thrombosis presents with acute onset of shortness of breath and left-sided chest pain that awoke her the morning of presentation. Her vital signs are significant for a heart rate of 106 beats/min, respiration rate of 22 breaths/min, blood pressure of 105/76 mm Hg, and pulse oximetry of 98% on room air. The patient denies previous exposure to oral contraceptives. She does not smoke. She reports that she had noticed left calf pain and swelling, which worsened with walking after a 4-hour drive 2 days prior.
What is the approach to diagnosis of thromboembolism in pregnant patients?
DEEP VEIN THROMBOSIS
Although a clinical diagnosis of DVT in pregnancy is unreliable, a history and physical examination are necessary to exclude other diagnoses and to assess the likelihood of thrombosis. Unfortunately, studies of the accuracy of history and physical examination for detecting DVT and PE have not included pregnant patients. In most pregnant patients with clinically suspected DVT, the diagnosis is not confirmed. Other causes of leg pain and swelling are not uncommon during pregnancy and include cellulitis, ruptured Baker’s cyst, or muscular pain.
A cross-sectional study described the derivation of the LEFt clinical decision rule, which relies on 3 variables in pregnant women with suspected DVT: left leg presentation (L), ≥ 2 cm calf circumference difference (E for edema), and first trimester presentation (Ft). If none of these variables is present, the negative predictive value is 100%. 16 A validation study suggested that a negative LEFt rule accurately identifies pregnant women in whom the risk for confirmed DVT appears to be very low. The rule should not be used as an individual test for excluding DVT during pregnancy, but could be applied in a diagnostic approach in association with D-dimer measurement and compression ultrasonography (CUS); however, it has not been prospectively validated for safety and efficacy. 17 In a study of 149 consecutive pregnant women with suspected DVT, a whole-blood agglutination D-dimer had a sensitivity of 100% and specificity of 60%. 18 A 2006 systematic review found only 4 diagnostic studies of VTE in pregnancy in the literature. One of these studies showed that a combination of a negative CUS and normal D-dimer can accurately exclude DVT. 19
Serial CUS is necessary for pregnant women with a high clinical suspicion of DVT but a negative initial investigation. In a study of 221 pregnant women in whom DVT was clinically suspected, 16 women (7.2%) were diagnosed with DVT by initial CUS, and none were diagnosed with DVT onserial testing. 20 During follow-up (≥ 3 months), 6 of the 205 women with normal serial CUS results presented with symptoms of DVT, PE, or both, and 1 of them was diagnosed with DVT and PE. The sensitivity of serial CUS with Doppler imaging was 94.1% (95% confidence interval [CI] 69.2% to 99.7%), and the negative predictive value was 99.5% (95% CI 96.9% to 100%). 20 All ultrasounds undertaken for investigation of pregnancy-associated DVT should include imaging of the iliac veins if there is a high index of suspicion and the CUS is negative for femoral DVT. Serial CUS with Doppler imaging of the iliac vein performed over a 7-day period excludes DVT in symptomatic pregnant women. 20 Repeat CUS may be done 2 to 4 days and 6 to 8 days after the initial scan.
Ileofemoral vein thrombosis accounts for approximately 90% of proximal thromboses in pregnancy, occurring most often in the left lower extremity. 20 The incidence of isolated iliac vein thrombosis in pregnancy is low, but when it does occur, delay in diagnosis can lead to significant morbidity. Therefore, for women with suspected isolated iliac vein thrombosis in whom CUS is negative or nondiagnostic, magnetic resonance direct thrombus imaging (MRDTI) should be performed. 21 Patients with iliac vein thrombosis may present with unexplained inguinal, pelvic, or abdominal pain, which may be accompanied by back pain, and they usually present with swelling of the entire leg. MRDTI does not require gadolinium contrast and its accuracy appears to be similar to that of venography for iliac vein thrombi in the nonpregnant population. 21 Exposure to gadolinium during pregnancy is associated with an increased risk for rheumatologic, inflammatory, or infiltrative skin conditions and stillbirth or neonatal death. 22
Ovarian vein thrombosis is a rare but serious diagnosis. It occurs mostly in the postpartum period, mainly after cesarean delivery, and usually affects the right ovarian vein. The diagnosis is confirmed by ultrasound, computed tomography (CT), or magnetic resonance imaging. 23
PULMONARY EMBOLISM
PE is more difficult to diagnose than DVT, particularly because clinical signs of PE are unreliable in the pregnant patient. The mortality rate of untreated PE is high, ranging from 18% to 38%, and approximately one-third of patients with untreated thromboembolic disease develop recurrent embolism. 24 Studies have reported a PE prevalence between 1.4% and 4.2% in pregnant women with suspected clinical diagnosis of PE. 25
The clinical presentation of PE and associated laboratory testing results may be subtler in pregnant than in nonpregnant patients. Arterial blood gases (ABG) may show hypoxemia or hypocapnia. The ABG in pregnancy has a sensitivity of 76.9%, specificity of 20.2%, and negative and positive predictive values of 80% and 11.5% for PE, respectively. 26 The alveolar-arterial oxygen gradient is a poor screening test for PE during pregnancy and postpartum. A retrospective chart review of 17 pregnant women with documented PE showed that 58% had normal alveolar-arterial gradients. 27 Therefore, in a pregnant woman with a history suspicious for PE, objective imaging studies should be performed even if the patient has normal ABG.
The 2011 guidelines from the American Thoracic Society (ATS) and the Society of Thoracic Radiology (STR) recommend against using D-dimer to diagnose PE in pregnancy. 28 In addition, lower extremity CUS should only be performed as the first diagnostic imaging procedure if the patient has signs or symptoms of DVT. Instead, the ATS/STR guidelines recommend a plain radiograph of the chest as the first imaging test. If the chest radiograph is normal, a ventilation/perfusion scan (V/Q) scan is preferred over CT pulmonary angiography (CTPA). Diagnostic accuracy of the V/Q scan may be superior to CTPA in pregnancy, and it is preferable because of the lower prevalence of indeterminate V/Q scan in pregnant women. 29 Moreover, there is lower radiation exposure to the maternal breast and lung tissue with a V/Q scan than with CTPA. CTPA confers lower fetal radiation doses than V/Q scans (0.03–0.66 mGy versus 0.32–0.74 mGy, respectively) but higher total body maternal radiation (4–16 mSv versus 1–2.5 mSv). 30 A quantitative approach to lung scan interpretation, based on the distribution histogram of V/Q ratios, may be helpful in categorizing patients with suspected PE. 28 A study of 121 suspected episodes of PE in 120 pregnant women showed that 104 women with normal or nondiagnostic scans did not develop subsequent episodes of VTE during a mean follow-up period of 20 months. 31
If the baseline chest radiograph is abnormal in a pregnant woman with clinical suspicion of PE, a CTPA should be performed. As noted, fetal radiation doses for CTPA examinations in which the fetus is not directly imaged are minimal. If CTPA is recommended for the diagnosis of PE, the patient should be informed that radiation to the breast may increase her baseline risk for breast cancer. The ATS guidelines state that “given the lack of evidence documenting clear superiority of any one diagnostic test, the values and preferences of a patient and her physician likely will and should determine the final choice and sequence of tests performed.” 28
CASE I CONTINUED
Upon presentation to the emergency department, the circumference of the patient’s left leg is not significantly greater than that of her right leg, and her leg pain has resolved. Bilateral CUS is negative for proximal or distal DVT. Chest radiograph shows an opacification of her left lower lobe. CTPA shows bilateral segmental and subsegmental lower lobe pulmonary emboli.
How does risk for VTE change throughout pregnancy?
Women are at increased risk for VTE throughout the entire pregnancy, starting from conception, but mainly during the postpartum period. A Danish historical controlled cohort study of 819,751 pregnant women (ages 15–49 years) over a 10-year period identified 727 women with VTE. The absolute risk for VTE per 10,000 pregnancy-years increased from 4.1 (95% CI 3.2 to 5.2) during weeks 1 to 11 to 59.0 (95% CI 46.1 to 76.4) in week 40 and decreased in the postpartum period from 60 (95% CI 47.2 to 76.4) during the first week after birth to 2.1 during weeks 9–12 after birth (95% CI 1.1 to 4.2). 32 This study showed that the risk of VTE increases throughout pregnancy and reaches its maximum during the peripartum period and is not significantly increased after 6 weeks post-delivery. In a retrospective cross-over cohort study of 1,687,930 women in California who delivered their first newborn, an elevated risk of VTE persisted until at least 12 weeks after delivery. However, the absolute increase in risk after 6 weeks postpartum was low. 33
CASE 1 CONCLUSION
The patient is started on anticoagulation therapy and carefully monitored during the remainder of the pregnancy and postpartum period. Anticoagulation is discontinued 6 weeks after delivery.
TREATMENT
ANTICOAGULATION THERAPY
The treatment of VTE can be lifesaving. In a study comparing 35 patients with PE randomly assigned to treatment with anticoagulants versus no treatment, 5 of 19 patients in the untreated group died from PE and an additional 5 had nonfatal recurrences, as compared with none in the treated group. 24 Unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) are both safe and effective anticoagulants during pregnancy as neither crosses the placenta. In a review of 186 reports of fetal and infant outcomes following anticoagulant therapy during pregnancy in 1325 pregnancies, outcomes in UFH-treated patients were similar to those in the normal population after excluding pregnancies with comorbid conditions independently associated with adverse outcomes. 34 A 2005 systematic review of LMWH for prophylaxis and treatment of VTE during pregnancy included 64 studies of 277 pregnancies. There were no maternal deaths, live births resulted from 94.7% of the pregnancies, VTE or arterial thrombosis occurred in 0.86%, and significant bleeding occurred in 1.98%. 35
The standard UFH regimen is an initial bolus of 5000 units subcutaneously and 17,500 units every 12 hours, with dose adjustment made based on a mid-interval activated partial thromboplastin time (aPTT). 36 Although still controversial, it has been suggested that the anti-Xa assay with a mid-dosing interval target of 0.3 to 0.7 U/mL is a more reliable measure of therapeutic UFH activity than the aPTT, as the aPTT response is suppressed due to a pregnancy-related increase in factor VIII. LMWH is dosed based on weight; regimens are enoxaparin 1 mg/kg subcutaneously twice daily or 1.5 mg/kg subcutaneously once daily, and dalteparin 100 units/kg every 12 hours or 150 units/kg daily.
A 2017 Cochrane review of the effect of LMWH compared with UFH for the treatment of VTE in the nonpregnant setting included 23 studies with 9587 patients. Thrombotic complications (odds ratio [OR] 0.70 [CI 0.57 to 0.85]) and major hemorrhage (OR 0.58 [CI 0.40 to 0.83]) were lower in patients receiving LMWH, with a trend toward lower mortality. 37 In addition, the incidence of bleeding complications in patients treated with subcutaneous LMWH versus intravenous heparin was compared in a 2012 systematic review of 27 randomized controlled trials with a total of 28,637 patients. In patients treated with LMWH, there was a nonstatistically significant lower incidence of major bleeding events (OR 0.79 [95% CI 0.60 to 1.04]) and a statistically significant reduction in bleeding risk (OR 0.68 [95% CI 0.47 to 1.00]) compared to patients treated with UFH. 38 Additionally, a trial comparing the use of standard UFH versus LMWH found a significantly lower incidence of thrombocytopenia in patients treated with LMWH. 39,40 Overall, LMWH is more effective at decreasing both thrombotic and bleeding complications, and the risk for osteoporosis is lower with LMWH. Based on these results, the American College of Chest Physicians (ACCP) recommends LMWH as the first-line treatment for VTE in pregnancy. 41
In specific clinical situations, such as patients with renal dysfunction with creatinine clearance (CrCl) less than 30 mL/min, UFH is indicated. In a study of 103 pregnancies in 93 women given anti-coagulation during pregnancy, 89.3% received UFH. There were no maternal deaths, and fetal demise occurred in 8 pregnancies (7.8%) at a median of 14 weeks’ gestation. There were 2 episodes of PE (1.9%) and 2 major bleeding events requiring transfusion (1.9%). 42 UFH costs much less than LMWH, and therefore UFH remains an important, inexpensive, and efficacious anticoagulant option for pregnant women who require anticoagulation and cannot afford LMWH. 43
Due to the physiologic changes associated with pregnancy, LMWH and UFH dosages may need to be adjusted. An observational study of 20 pregnant women with acute VTE found no recurrent VTE or major bleeding after treatment with dalteparin. Dalteparin doses approximately 10% to 20% higher than those recommended in nonpregnant women were required to reach therapeutic target anti-Xa activity. 44
Caution Regarding Oral Anticoagulants
Due to its teratogenicity, warfarin is not a first-line anticoagulation option. It is strictly contraindicated during the first trimester during organogenesis, and its use during pregnancy is restricted to women with mechanical heart valves. Warfarin crosses the placenta and has been associated with nasal hypoplasia, stippled epiphyses, and growth restriction, particularly between 6 to 9 weeks’ gestation. Every effort should be made to substitute UFH or LMWH for warfarin between 6 and 12 weeks of gestation. The bridging process should begin as early in the gestational age as possible due to the long half-life of warfarin. 45 When used later in gestation, warfarin has been associated with fetal hemorrhage and central nervous system abnormalities. Other complications from use during the second and third trimesters include microcephaly, blindness, deafness, and fetal growth restriction. 46,47 Its use also increases the risk for abortion and fetal death in utero. 48–50
The direct oral anticoagulants (DOACs) are not approved for use in pregnancy. Although there are limited anecdotal reports of DOAC use in pregnancy, 51 there is preclinical evidence of placental transfer with the DOACs rivaroxaban and apixaban (direct Xa inhibitors) and the oral thrombin inhibitor dabigatran, thus increasing the risk to the fetus. 52–54 Edoxaban, another direct Xa inhibitor, should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It should be discontinued in nursing mothers. 55
THROMBOLYSIS
Fetal as well as maternal survival is dependent on adequate maternal perfusion and oxygenation. The risk of death from PE is significant, with a cross-sectional study of 58 patients with acute, massive PE showing a 55% mortality rate. 56 Thus, pregnancy is not an absolute contraindication to mechanical or systemic (recombinant tissue plasminogen activator or streptokinase) thrombolysis in an unstable patient at high risk for death. 57–59 There are no major studies of this approach, although a small review of 13 cases using systemic thrombolysis showed no increased risk of maternal mortality. 58 Thrombolysis should be considered for appropriate indications in pregnant patients as it would be in nonpregnant patients. However, caution is required when drawing conclusions regarding maternal and fetal safety, given the lack of controlled clinical trials including pregnant women.
SURGICAL PULMONARY EMBOLECTOMY
Surgical pulmonary embolectomy is an important therapeutic and potentially life-saving option in women presenting with massive PE in the immediate postpartum period. Because of the risk of massive uterine bleeding immediately postpartum, thrombolytic therapy should not be used. 60
INFERIOR VENA CAVA FILTER
Placement of an inferior vena cava (IVC) filter is indicated in patients who have an acute VTE with absolute contraindications for anticoagulation. In addition, it can be considered in patients with extensive ileofemoral venous thrombosis within 2 weeks prior to expected delivery. 61 In a systematic review of 44 studies of IVC filters placed in pregnant patients, the IVC filter complication rate was 8.87% and the failure-to-retrieve rate was 11.25%. 62 The complication rate is similar to that found in the nonpregnant population. Thus, IVC filters may be used when appropriately indicated and should be removed as soon as clinically feasible.
RECURRENT THROMBOSIS AND THROMBOPHILIAS
CASE PRESENTATION 2
A 34-year-old pregnant woman G1P0 at 38 weeks’ gestation presents with a painful, swollen left calf that is associated with difficulty on walking; the circumference of the left calf is 2 cm greater than that of the right. She has no shortness of breath or chest pain. She has a prior history of distal right lower extremity DVT while on combined oral contraceptives. Her mother also has a history of DVT while bedbound during a prolonged hospitalization at an older age. CUS is negative, and the patient is discharged home. However, 24 hours later she returns to the hospital with worsening swelling and pain in her left leg. Magnetic resonance venography demonstrates a large left external iliac and common iliac DVT. She is admitted and is started on UFH, and a retrievable IVC filter is placed in anticipation of delivery.
What is the risk for VTE recurrence during pregnancy?
A personal and family history of VTE should be obtained when evaluating pregnant patients. A retrospective study of 109 women with prior history of VTE showed recurrence rates per patient-year of 10.9% during pregnancy and 3.7% in the nonpregnant period; the relative risk of recurrent VTE during pregnancy was 3.5 (95% CI 1.6 to 7.8). 63 Two large European retrospective cohort studies of VTE in pregnancy showed that the recurrence rate of VTE in women with a history of thrombosis is around 6% during pregnancy, equally distributed among trimesters. The highest incidence of recurrence was in the postpartum period, ranging from 8.3% to 10%. 64 The recurrence risk during pregnancy in women with a history of a single episode of VTE was 2.4% antepartum (95% CI 0.2% to 6.9%). 65 These risks may be lower in women without thrombophilia or with a temporary risk factor associated with their previous thromboembolic event. 65 Recurrence risk is higher if the previous VTE was estrogen-related, either due to pregnancy or through hormonal contraception (10%), than if the previous VTE was non-estrogen-related (2.7%). 64,66
The timing of the case patient’s presentation is consistent with reports of increased risk of VTE during the peripartum period. Her prior history of estrogen-related DVT is concerning for a risk of recurrence, particularly during pregnancy. A retrospective cohort study of 1104 women with previous VTE, 88 of whom became pregnant without receiving thromboprophylaxis, showed that the overall rate of VTE recurrence was 5.8% (95% CI 3.0% to 10.6%) and 8.3% (95% CI 4.5% to 14.6%) during pregnancy and postpartum, respectively. The risk of VTE recurrence was absent if the first VTE was related to a transient risk factor other than pregnancy, postpartum period, or hormonal contraception. 67 However, the recurrence rate of VTE in women with prior unprovoked VTE and/or thrombophilia has been reported as 5.9% (95% CI 1.2% to 16.2%). 65 The presence of an underlying hypercoagulable state can increase the recurrence risk by 25% to 50%, depending on the disorder. 68 A retrospective cohort study of 270 pregnancies in 105 carriers of factor V Leiden, identified because of a symptomatic relative with the factor V Leiden mutation, found a VTE risk (mostly in the postpartum period) of 6.4% for heterozygous women, 16.7% for homozygous women, 20% for double heterozygous women, and 1.2% for noncarriers. 69
Should the patient be screened for a thrombophilia disorder?
Half of all index thromboses in patients with thrombophilia occur in association with an additional risk factor. In women of child-bearing age, pregnancy, the postpartum period, and the use of combined hormonal contraception are all risk factors for VTE. A 2010 guideline from the British hematology community recommended testing for thrombophilia in women with prior VTE secondary to a minor provoking factor before or during pregnancy, but not testing women with unprovoked VTE (who would receive prophylaxis regardless) or those with VTE secondary to a major provoking factor (who would not require prophylaxis). 70 Indications to screen for aPL antibodies include: women with (1) 3 unexplained recurrent first-trimester pregnancy losses or 1 second or third trimester fetal loss of morphologically normal fetuses; (2) severe preeclampsia; (3) intrauterine growth restriction; or (4) premature labor (< 34 weeks’ gestation). 71,72
CASE 2 CONCLUSION
The patient is subsequently screened for inherited thrombophilia disorders and is found to be heterozygous for factor V Leiden.
CASE PRESENTATION 3
A 25-year-old woman is diagnosed with antiphospholipid syndrome (APS) during her second pregnancy when she experiences fetal loss during her second trimester. Pathologic examination of the placenta reveals infarcts. Laboratory evaluation reveals positive high-titer anticardiolipin and anti-beta-2 glycoprotein 1 antibodies (IgG isotype) and lupus anticoagulant on 2 separate occasions 12 weeks apart. In a subsequent pregnancy, she is started on prophylactic LMWH and daily low-dose aspirin (81 mg). At 36 weeks’ gestation, she presents with a blood pressure of 210/104 mm Hg and a platelet count of 94,000 cells/ µL. She is diagnosed with preeclampsia and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome and is induced for early delivery. About 2 weeks after vaginal delivery, she notices shortness of breath and chest pain. A CTPA demonstrates a right lower lobe lobar defect consistent with a PE. Her anticoagulation is increased to therapeutic dosage LMWH.
To what extent does thrombophilia increase the risk for VTE in pregnancy?
Approximately 50% of pregnancy-related VTEs are associated with inherited thrombophilia. A systematic review of 79 studies, in which 9 studies ( n = 2526 patients) assessed the risk of VTE associated with inherited thrombophilia in pregnancy, revealed that the odds ratio for individuals with thrombophilia to develop VTE ranged from 0.74 to 34.40. 73 Although women with thrombophilia have an increased relative risk of developing VTE in pregnancy, the absolute risk of VTE remains low ( Table 1 ).41,73,74
How is APS managed in pregnant patients?
Women with history of recurrent early pregnancy loss (< 10 weeks’ gestation) related to the presence of aPL antibodies are managed with low-dose aspirin and prophylactic-dose UFH or LMWH. This treatment increases the rate of subsequent successful pregnancy outcomes and reduces the risk for thrombosis. A 2010 systematic review and meta-analysis of UFH plus low-dose aspirin compared with low-dose aspirin alone in patients with APS and recurrent pregnancy loss included 5 trials and 334 patients. Patients receiving dual therapy had higher rates of live births (74.3%; relative risk [RR] 1.30 [CI 1.04 to 1.63]) compared to the aspirin-only group (55.8%). 75 A 2009 randomized controlled trial compared low-dose aspirin to low-dose aspirin plus LMWH in women with recurrent pregnancy loss and either aPL antibodies, antinuclear antibody, or inherited thrombophilia. The study was stopped early after 4 years and found no difference in rates of live births between the groups (77.8% versus 79.1%). 76 However, a randomized case-control trial of women with aPL antibodies and recurrent miscarriage found a 72% live birth rate in 47 women randomly assigned to low-dose aspirin and LMWH. 77 A 2012 guideline from the American College of Chest Physicians (ACCP) recommends that women with aPL antibodies with a history of 3 or more pregnancy losses receive low-dose aspirin plus prophylactic-dose LMWH or UFH. 78 A 2014 systematic review and meta-analysis showed that the combination of low-dose aspirin and UFH resulted in a higher live-birth rate than aspirin alone in 803 women with APS (RR 1.54 [95% CI 1.25 to 1.89]). 79 Further large randomized controlled trials are needed to confirm optimal management of recurrent miscarriage and aPL antibodies.
The addition of prednisone to aspirin, heparin, or both has shown no benefits in pregnant women with aPL antibodies. Indeed, prolonged use of steroids may cause serious pregnancy complications, such as prematurity and hypertension. 80–83 Intravenous infusions of immunoglobulin (IVIG) have not been shown to be superior to heparin and aspirin. This finding was confirmed in a multicenter clinical trial that tested the effects of IVIG compared with LMWH plus low-dose aspirin for the treatment of women with aPL antibodies and recurrent miscarriage. The rate of live-birth was 72.5% in the group treated with heparin plus low-dose aspirin compared with 39.5% in the IVIG group. 84
Preeclampsia and HELLP syndrome complicated the case patient’s pregnancy even though she was being treated with prophylactic-dose LMWH and low-dose aspirin, the current standard of care for pregnant women with APS (UFH can be used as well). It is important to note that complications may still occur despite standard treatment. Indeed, PE is more common in the postpartum than in the antepartum period. Prompt diagnosis is paramount to initiate the appropriate treatment; in this case the dose of LMWH was increased from prophylactic to therapeutic dose. However, additional therapeutic modalities are necessary to improve outcomes. A randomized controlled trial comparing standard of care with or without hydroxychloroquine is under way to address this issue.
PROPHYLAXIS
CASE PRESENTATION 4
A 34-year-old woman G1P0 at 6 weeks’ gestation with a past medical history of a proximal lower extremity DVT while on oral contraception is treated with warfarin anticoagulation for 6 months. Her obstetrician consults the hematologist to advise regarding antithrombotic management during this pregnancy.
What is the approach to prophylaxis in women at high risk for pregnancy-associated VTE?
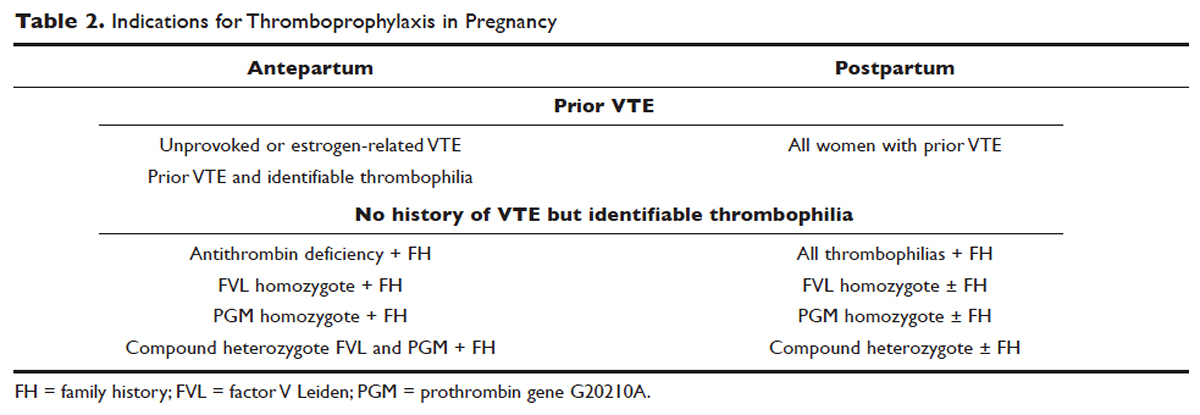
All women at high risk for pregnancy-associated VTE should be counseled about the signs and symptoms of DVT or PE during preconception and pregnancy and have a plan developed should these symptoms arise. The ACCP guidelines on antithrombotic therapy outline recommendations ranging from clinical vigilance to prophylactic and intermediate-dose anticoagulation, depending on the risk for VTE recurrence, based on the personal and family history of VTE and type of thrombophilia ( Table 2 ).78 These recommendations range from grade 2B to 2C.
For women with a history of estrogen-related VTE, single unprovoked VTE, or recurrent unprovoked VTE not on chronic anticoagulation, antepartum and postpartum pharmacologic thromboprophylaxis with either prophylactic or intermediate-dose LMWH is recommended (grade 2C). In patients with prior history of provoked VTE (non-estrogen related), antepartum clinical vigilance and postpartum pharmacologic thromboprophylaxis is recommended (grade 2C, 2B).
In asymptomatic pregnant women who are homozygote carriers for factor V Leiden or prothrombin G20210A variants and have a positive family history of thrombosis, antepartum and postpartum pharmacologic thromboprophylaxis is recommended (grade 2B). In asymptomatic homozygote carriers of factor V Leiden or prothrombin G20210A variants with no family history of thrombosis and women with all other thrombophilias with a positive family history of thrombosis, postpartum pharmacologic thromboprophylaxis is indicated (grade 2B and 2C, respectively). For women with confirmed APS and clinical criteria of obstetric APS with recurrent pregnancy loss, antepartum thromboprophylaxis with LMWH and low-dose aspirin is recommended (grade 1B). For pregnant women with all other thrombophilias with no personal or family history of thrombosis, clinical vigilance is suggested (grade 2 C). 78
As an alternative to LMWH, vitamin K antagonists (VKA) such as warfarin can be used for postpartum thromboprophylaxis; in patients with protein C or S deficiency, due to the risk of warfarin-induced skin necrosis, a rapid-onset anticoagulant must be concomitantly administered. Warfarin and LMWH are safe anticoagulants during lactation, but there are no clinical data on the effects of the DOACs on infants during lactation. Data from animal studies indicate that DOACs are secreted into breast milk. 85
What risks are associated with anticoagulant therapy in pregnancy?
VKAs cross the placenta and can cause teratogenicity, pregnancy loss, fetal bleeding, and neurodevelopmental deficits. Therefore, discontinuation of VKAs prior to the sixth week of gestation is necessary to avoid warfarin embryopathy. DOACs have been shown to readily cross the placenta but with unknown human reproductive risks. Fondaparinux, a synthetic pentasaccharide, crosses the placenta in small quantities. Though there are reports of the successful use of fondaparinux in pregnancy, there is limited reported experience of its use in the first trimester. 86
The risk for bleeding with anticoagulation is notably acceptable. In a case-control study of 88 pregnant women receiving therapeutic-dose anticoagulation, the risk of postpartum hemorrhage (PPH) after vaginal delivery was 30% in those who received LMWH anticoagulation versus 18% in those who did not (OR 1.9 [95% CI 1.1 to 3.5]). 87 However, the risk for severe PPH (≥ 500 mL) was similar (5.6% versus 5.0%; OR 1.1 [95% CI 0.4 to 3.6]). The risk for PPH after cesarean section was 12% in LMWH users versus 4% in LMWH non-users (OR 2.9 [95% CI 0.5 to 19.4]). The risk for PPH associated with delivery within 24 hours after the last dose of LMWH was 1.2 times higher (95% CI 0.4 to 3.6) compared to a longer interval. Therefore, therapeutic LMWH increases the risk for blood loss after vaginal delivery, but not the risk for severe PPH. The risk for PPH is influenced by the interval between the last dose of LMWH and delivery. Of note in this study, per the institution’s protocol, the anticoagulation was stopped with signs of labor or determination of need for delivery. The risk for blood loss may be mitigated in more planned delivery scenarios. 87
CASE 4 CONTINUED
The patient is placed on prophylactic-dose LMWH with good tolerance and delivers at 39 weeks' gestation via caesarian section due to nonprogression of labor. Postpartum she is restarted on prophylactic-dose anticoagulation with LMWH. Two weeks after discharge from the hospital, she presents with right calf pain and mild shortness of breath. On physical exam, her leg circumferences are equal. A D-dimer assay is 3375 ng/mL (normal 0–229). CUS of the right leg shows a complete occlusive DVT of the mid-distal superficial femoral and popliteal veins and partially occlusive acute DVT of the right posterior tibial and peroneal veins. CTPA reveals a right lower lobe PE. Because she had developed VTE despite prophylactic LMWH, her anticoagulation is changed to therapeutic dose. She is treated with anticoagulation with LMWH for a total of 3 months, after which a repeat CUS shows no residual thrombosis.
What is the recommended dosing of heparin and LMWH during pregnancy?
A prospective study of 14 pregnant women receiving UFH prophylaxis found that a prophylactic dose of 5000 units twice a day was inadequate to achieve prophylactic heparin levels in any patient in the second or third trimester. 88 Similar to treatment dosage, there is no consensus evidence for prophylactic dosing, and dosage recommendations are based on expert opinion. In a retrospective study of 25 pregnant women on intermediate-dose UFH, the mean UFH dose required to achieve a target anti-factor Xa level of 0.1 to 0.3 units/mL was 236.9 units/kg/day. 89 However, the use of anti-factor Xa levels for monitoring is controversial as there is no data to support a difference in outcomes with its use in prophylactic or therapeutic dosing.
The timing of the previous VTE history is important when deciding on the anticoagulant dose in pregnancy. In pregnant women with a VTE that occurred within the previous 4 to 6 weeks, full-dose anticoagulation with LMWH should be considered; an intermediate dose (three-fourths of a therapeutic dose) may be used if the thrombotic episode occurred more than 6 weeks earlier but still within a year. Prophylactic dosing may be sufficient if the episode occurred more than a year earlier. 90 A clinical trial (High-Low) is under way to explore the optimal dose of LMWH in pregnant women with prior history of VTE who are not on chronic anticoagulation therapy. 91
How is anticoagulation therapy managed in the peripartum period?
Neuraxial anesthesia during active labor while on anticoagulation increases the risk for central nervous system bleeding. Therefore, if spontaneous labor occurs in women on therapeutic dose anticoagulation, neuraxial anesthesia cannot be used. However, in the event of elective induction of labor or caesarean section, neuroaxial anesthesia may be performed 12 hours after the administration of the last prophylactic dose of LMWH or 24 hours after the last therapeutic dose of LMWH. Intravenous UFH should be stopped for 6 hours before induction of labor with a confirmed normal aPTT before placement of neuraxial anesthesia. There is no contraindication for using neuraxial anesthesia during subcutaneous standard UFH at total doses of 10,000 units daily. The risk of spinal hematoma with larger daily subcutaneous doses is unclear; therefore, a documented normal aPTT must be obtained before placement of neuroaxial anesthesia.
Postpartum, reinitiation of prophylactic-dose LMWH should be delayed for at least 12 hours after the removal of an epidural catheter. Therapeutic-dose LMWH should be administered no earlier than 24 hours after neuraxial anesthesia, providing that proper hemostasis is achieved. In the absence of persistent bleeding, if no regional anesthesia was used, LMWH may be resumed 12 hours after delivery. 92 Anticoagulation with either LMWH or warfarin is recommended for at least 6 to 12 weeks postpartum. 33
COUNSELING
Patients should be advised to manage controllable risk factors, including avoiding prolonged immobilization, avoiding excessive weight gain in pregnancy, and stopping smoking. Periods of immobilization tend to cause reduced blood flow (stasis), which predisposes to thrombosis. In a systematic review of records of all patients with confirmed PE after arrival at Charles de Gaulle airport in Paris during a 13-year period, women had a higher risk of PE after a long-distance flight than men, with an estimated incidence of 0.61 per million passengers versus 0.20, respectively; the incidence reached 7.24 and 2.35 cases, respectively, in passengers traveling more than 10,000 kilometers. 93,94
The risk of air travel-related thrombosis in pregnant women is estimated to be between 0.03% and 0.1%. Physicians must decide on an individual basis how to prevent travel-related thrombosis in their pregnant patients. In most passengers, prevention can be limited to encouraging exercise, avoidance of long sleeping periods, and not using a window seat. Women at high risk for VTE, such as women with a prior history of VTE who are not on anticoagulation or women with known asymptomatic thrombophilia or other risk factors for thrombosis such as obesity, may benefit from a short period (1–3 days) of LMWH starting 2 hours before a long-distance flight. 95
Activation of the coagulation system has been demonstrated in cigarette smokers. 96 Heavy smoking was found to be a significant risk factor for VTE in a cross-sectional analysis of 2404 men and women. 97 An increased risk for thrombosis during pregnancy is seen in cigarette smokers 15,98 and is enhanced with the concomitant use of illicit drugs. 99 Other obstetric complications associated with smoking and illicit drug use during pregnancy include preterm labor, spontaneous abortion, perinatal death, low birth weight, and abruption placenta. The efficacy of nicotine replacement therapy in pregnancy is uncertain. 100 Recommendations are to advise patients to stop smoking, obtain psychosocial counseling, and utilize adjunctive therapies, which have been shown to have some effect on abstinence rates. 101
CONCLUSION
Women are at increased risk for VTE during pregnancy and the postpartum period. Awareness of risk factors and the signs and symptoms of VTE is paramount. Prompt diagnosis and treatment is mandatory to decrease complications of VTE. LMWH is the mainstay treatment of VTE in pregnancy, as it does not cross the placenta. Both LMWH and warfarin are safe during lactation. Close communication among the patient, obstetrician, hematologist, anesthesiologist, and neonatologist is crucial to optimize the care of these patients.