Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
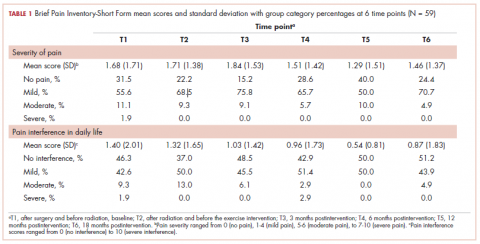
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
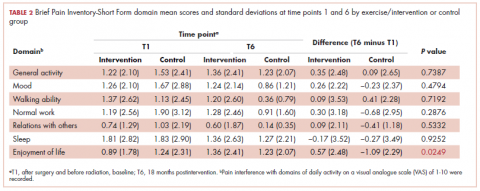
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
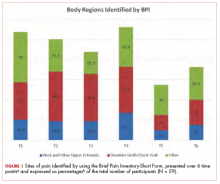
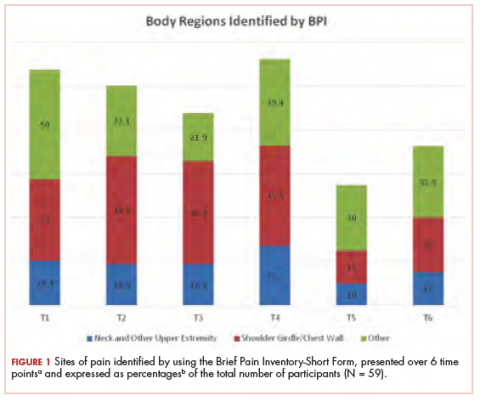
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
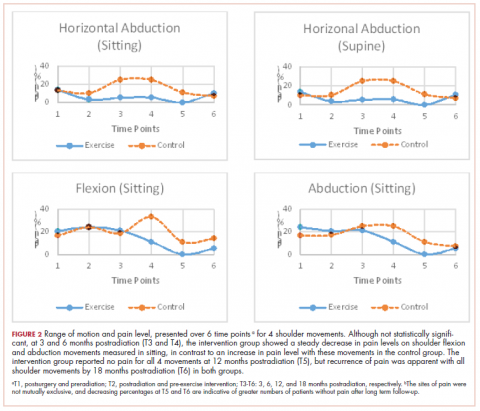
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.