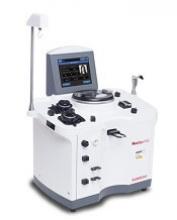
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Haemonetics Corporation’s NexSys PCS™ plasmapheresis system.
The open architecture of NexSys PCS facilitates bi-directional connectivity to donor management systems, enabling automated collection procedure programming and automated end-of-procedure documentation.
The guided operation, large touch screen, and on-screen troubleshooting assistance on NexSys PCS are designed to improve plasma center efficiency. The goal is to reduce donors’ time in centers and improve the centers’ collection capacity.
“NexSys PCS is designed to increase productivity and improve quality and compliance in plasma collection centers,” said Christopher Simon, CEO of Haemonetics.
“Each of these benefits is noteworthy and, when combined, we believe will unlock meaningful value for our customers.”
Haemonetics plans to begin limited production of NexSys PCS devices immediately and to pursue further regulatory clearances for additional enhancements to the overall product offering.
NexSys PCS was previously referred to as PCS 300.