The telomerase inhibitor imetelstat has exhibited unique activity in a pilot study of patients with intermediate- or high-risk myelofibrosis (MF), but more research is needed, according to investigators.
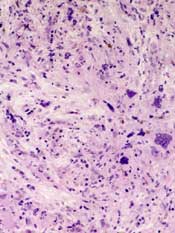
Imetelstat produced complete and partial responses in a minority of patients and reversed bone marrow fibrosis in complete responders.
However, imetelstat also prompted severe myelosuppression and liver-test abnormalities. And most patients ultimately discontinued treatment.
The investigators therefore concluded that additional research is needed to establish the most effective dosing of the drug, clarify its mechanism of action, and address concerns about toxicity.
Ayalew Tefferi, MD, of the Mayo Clinic in Rochester, Minnesota, and his colleagues reported the results of this trial in NEJM.
A phase 2 trial of imetelstat in patients with essential thrombocythemia was published in NEJM simultaneously. Both trials were sponsored by Geron Corporation, the company developing imetelstat.
The MF study included 33 patients, 18 with primary MF, 10 with post-polycythemia vera MF, and 5 with post-essential thrombocythemia MF. About 52% had high-risk disease, and the rest had intermediate-2-risk MF.
The patients had a median age of 67. About 79% of patients had received prior therapy, and 48% had received a JAK inhibitor. Thirty-nine percent of patients were dependent on red cell transfusions, 64% had constitutional symptoms, 70% had palpable splenomegaly, and 55% had an abnormal karyotype, including 18% with an unfavorable karyotype.
Imetelstat was administered as a 2-hour intravenous infusion, at a starting dose of 9.4 mg per kilogram of body weight. There were 2 dosing schedules: (1) once every 3 weeks or (2) weekly for 4 weeks, followed by once every 3 weeks.
Responses
“We observed that imetelstat was active and induced morphologic and molecular remissions in some patients with myelofibrosis,” Dr Tefferi said. “We also observed that imetelstat demonstrated selective anticlonal activity, inhibiting the growth of cancer cells, which we had not previously documented with other drugs.”
Overall, 21% of patients (7/33) experienced a complete response (n=4) or partial response (n=3) to treatment. The median duration of complete response was 18 months (range, 13-20+), and the median duration of partial response was 10 months (range, 7-10+).
“Some patients treated with imetelstat have reverted back to normal bone marrow,” Dr Tefferi noted. “Typically, myelofibrosis is characterized by marrow scarring, and, although patients may derive symptomatic relief from other treatments, such as ruxolitinib, they usually do not revert back to normal bone marrow.”
Bone marrow fibrosis was reversed in all 4 patients with a complete response. A molecular response was documented in 3 of these patients.
Mutations and telomere length
“We noted a difference in response rates, especially in complete remission rates, in patients with and without certain specific gene mutations, such as ASXL1, SF3B1, and U2AF1,” Dr Tefferi noted. “This underscores the need for laboratory correlative studies in future clinical trials.”
Responses occurred in 27% of patients with a JAK2 mutation and 0% of patients without a JAK2 mutation (P=0.30). Alternatively, responses occurred in 32% of patients without an ASXL1 mutation and 0% of patients with an ASXL1 mutation (P=0.07).
The rate of complete response was 38% among patients with a mutation in SF3B1 or U2AF1, compared to 4% among patients without either mutation (P=0.04).
The investigators also found that responses were not correlated with baseline telomere length.
Toxicity
Dr Tefferi and his colleagues said the most clinically significant side effect of imetelstat was myelosuppression. It was the primary reason for a protocol-mandated dose reduction that occurred in 22 patients (67%).
Another “notable” side effect was the elevation of liver-enzyme levels. The investigators observed treatment-emergent (though not necessarily related) increases from baseline in total bilirubin (49%), alkaline phosphatase (58%), aspartate aminotransferase (58%), and alanine aminotransferase (27%).
None of these abnormalities were linked to clinically overt liver damage, and most patients ultimately saw their values return to baseline levels.
Adverse events that were considered at least possibly related to treatment and occurred in 3 or more patients included thrombocytopenia (45% grade 3/4), anemia (39% overall, 30% grade 3), neutropenia (27% grade 3/4), aspartate aminotransferase elevation (27% grade 1), alkaline phosphatase elevation (21% grade 1/2), elevation in total bilirubin (12% grade 1/2), infusion-related reactions (12% grade 1/2), diarrhea (9% grade 1/2), and epistaxis (9% grade 1/2).
Treatment discontinuation
At the data-cutoff date (December 5, 2014), 76% of patients had discontinued imetelstat (n=25). For all patients, the median duration of treatment was 8.6 months (range, 1.4 to 21.7).
Patients stopped treatment due to insufficient response (n=16), disease progression or relapse after response (n=3), death during the treatment period (n=2), adverse events (n=2), financial constraints (n=1), and pre-existing atrial fibrillation (n=1).
Both patients who discontinued imetelstat due to adverse events had persistent thrombocytopenia. Of the 2 deaths, 1 was considered treatment-related. That patient died of intracranial hemorrhage that was attributed to drug-induced, grade 4 thrombocytopenia after weekly dosing. The non-treatment-related death was the result of an upper gastrointestinal hemorrhage.


