Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying, pulmonary, renal, and liver disease, and blood disorders.
Patients with an IMFS, (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, in patients with IMFS, classic phenotypical findings may be lacking in up to 30% to 40% of patients.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
Differential Diagnosis
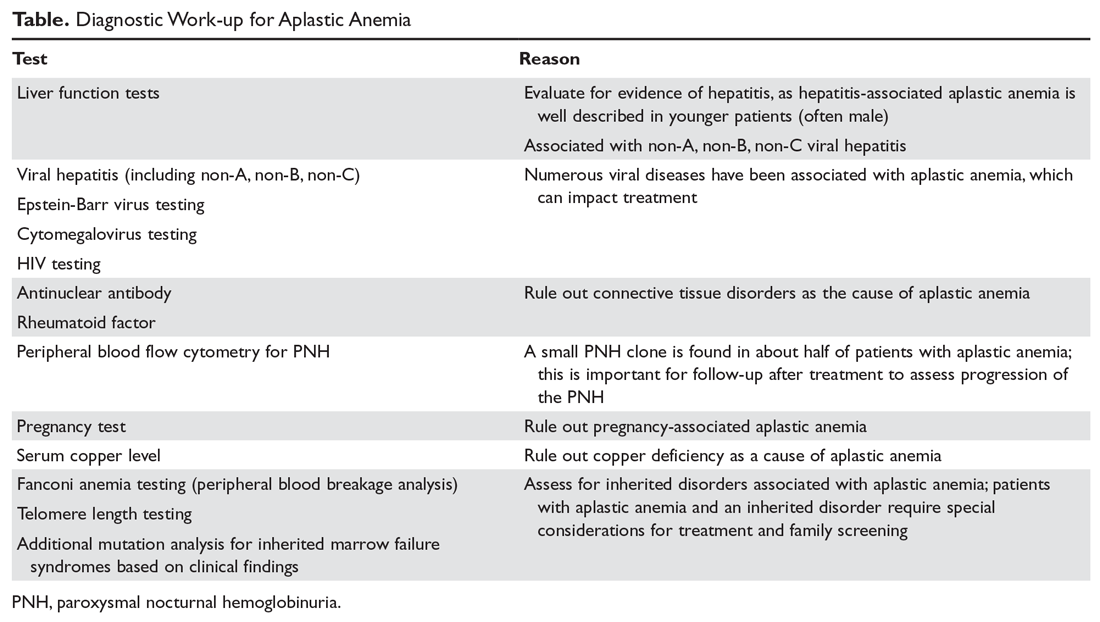
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7
Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 The typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35