Treatment of Metastatic CRPC (M1 Disease)
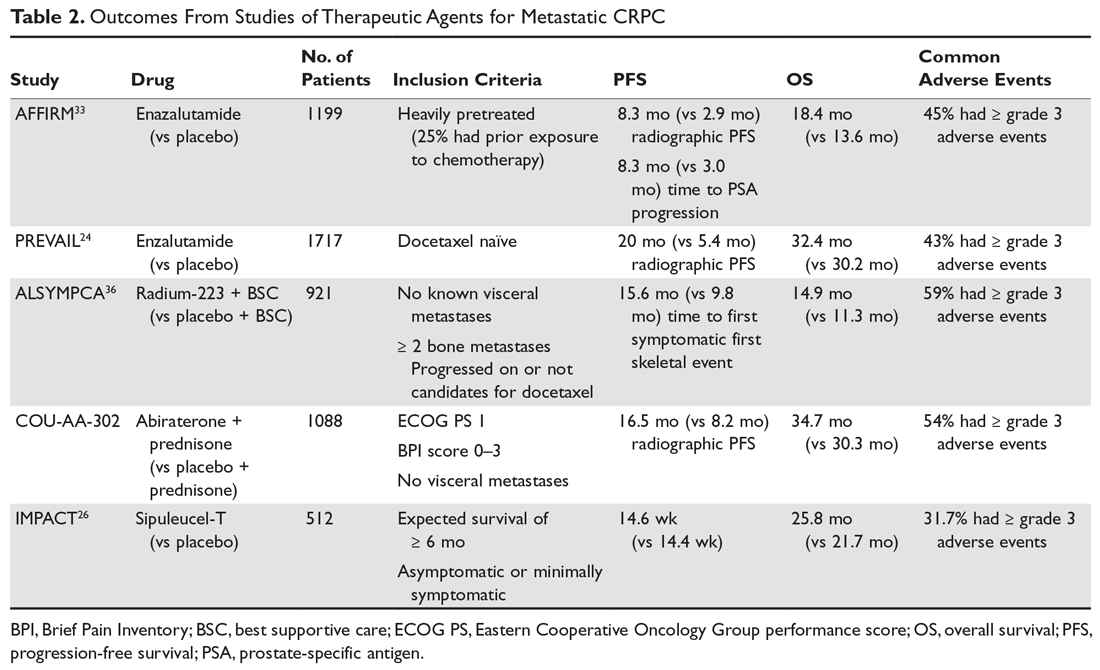
As with M0 CRPC, ADT should be continued in patients with mCRPC to maintain castration levels of testosterone while initiating additional treatments. Several drugs for the treatment of mCRPC have been approved by the US Food and Drug Administration (FDA) since 2010, including abiraterone with prednisone (or methylprednisolone), enzalutamide (but not apalutamide), radium-223, sipuleucel-T, and cabazitaxel (Table 2).
Given the availability of numerous treatment options for men with mCRPC, the sequencing of treatments should be based on careful consideration of the efficacy and adverse effect profiles of each drug as well as the anatomic and molecular characteristics of the cancer, comorbidities, and patient preference. If there is no evidence of visceral disease and the patient has an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 with an estimated life expectancy of greater than 6 months and is minimally symptomatic, then treatment with either oral targeted agents or immunotherapy with sipuleucel-T is considered appropriate.
Sipuleucel-T is an autologous dendritic cell vaccination designed to enhance T-cell–mediated response to prostatic acid phosphatase (PAP). The treatments are prepared from leukapheresed host mononuclear cells that are then exposed to PAP fused to granulocyte-macrophage colony-stimulating factor. The activated dendritic cells are then infused back into the host once every 2 weeks for a total of 3 treatments. The main side effects of this treatment include chills, fever, and headache, but it is generally well-tolerated and has demonstrated a survival benefit.26
Both enzalutamide and abiraterone (abiraterone given with physiologic-dose steroid replacement) confer a survival benefit in chemotherapy-naive patients with M1 CRPC. Per the PREVAIL study, enzalutamide (when compared to placebo) offers a median improvement in overall survival (OS) by about 2 months and in radiographic PFS by about 14.6 months.24 Abiraterone blocks the synthesis of androgens via inhibition of CYP17 in the testes and adrenal glands. Abiraterone also confers an overall survival advantage for patients with M1 CRPC who are chemotherapy-naïve, with an estimated 25% decrease in the risk of death (hazard ratio, 0.75, P = 0.009) when compared to prednisone.27
In patients with symptomatic M1 CRPC who have visceral disease or rapidly progressive disease and who are candidates for chemotherapy, docetaxel is frequently used and is given concurrently with steroids. Docetaxel has been given for up to 10 cycles in clinical trials (assuming no progression of disease or dose-limiting toxicities were observed), and at least 6 cycles of treatment are recommended. When compared to mitoxantrone plus prednisone in the TAX 327 phase 3 trial, docetaxel plus prednisone offered a significant OS benefit of about 3 months (19.2 months versus 16.3 months).28 For patients who are not candidates for docetaxel (eg, due to preexisting peripheral neuropathy), cabazitaxel should be considered. OS is similar for mCRPC with docetaxel versus cabazitaxel when given in the first-line setting.29 Additionally, cabazitaxel dosed at 20 mg/m2 is noninferior to cabazitaxel dosed at 25 mg/m2, and the lower dose is associated with lower rates of peripheral neuropathy.30 Cabazitaxel should also be considered for mCRPC that has progressed following treatment with docetaxel, with improved OS and PFS when compared to treatment with mitoxantrone and prednisone in this setting, as shown in the TROPIC study.31 Mitoxantrone given with prednisone has been shown to improve quality of life, but it is associated with significant cardiac toxicity. Additionally, mitoxantrone does not improve disease-free survival or OS in chemotherapy-naive patients32 or in patients who have progressed on docetaxel, and therefore should not be given to patients prior to a taxane chemotherapy unless the patient absolutely cannot tolerate docetaxel or cabazitaxel.
Once a patient’s prostate cancer progresses following treatment with a taxane, the sequence in which to administer subsequent therapies should involve careful consideration of previous treatments and duration of response to each of these treatments. Both enzalutamide and abiraterone are FDA-approved for use following treatment with chemotherapy. Per the AFFIRM trial, heavily pre-treated patients (including those who have received docetaxel) have a median 5-month OS benefit with enzalutamide compared to placebo.33 Another study of M1 CRPC patients who had previously received docetaxel demonstrated an OS benefit with abiraterone (versus placebo),34 but this regimen has limited benefit in patients who have previously received both docetaxel and enzalutamide.35 A rechallenge with docetaxel therapy also can be considered if the patient’s disease responded to docetaxel in the metastatic hormone-sensitive setting.
If the patient’s metastases are limited to bone (ie, no visceral disease), then radiotherapy with radium-223 should be considered. Radium-223 is an alpha-emitting calcium-mimetic radioactive compound that tracks to bone to delay the onset of symptoms from bone metastases.36 Radium-223 also confers a median OS benefit of about 3 months.37 However, this treatment is often limited by preexisting cytopenias.
Diethylstilbestrol (1 mg daily) competes with androgens for AR binding and is also cytotoxic to androgen-sensitive and insensitive prostate cancer cells. While its efficacy is similar to bicalutamide in terms of PSA response rate and median response duration, diethylstilbestrol is associated with significantly more cardiovascular toxicity, including stroke, pulmonary embolism, and heart failure, and its use is therefore limited.38 The glucocorticoids—prednisone (5 mg orally twice daily), dexamethasone (0.5 mg daily), and hydrocortisone (40 mg daily)—inhibit pituitary synthesis of adrenocorticotropic hormone, resulting in decreased adrenal androgen synthesis. Data suggest that among the glucocorticoids, dexamethasone monotherapy may produce superior response rates compared to prednisone monotherapy.39 While the glucocorticoids do produce a PSA response, prolong time to disease progression, and can provide symptomatic relief (eg, from bone pain), they have not been shown to confer a survival benefit and therefore are not commonly used as monotherapy.