Combination CTLA-4 and PD-1 Therapy
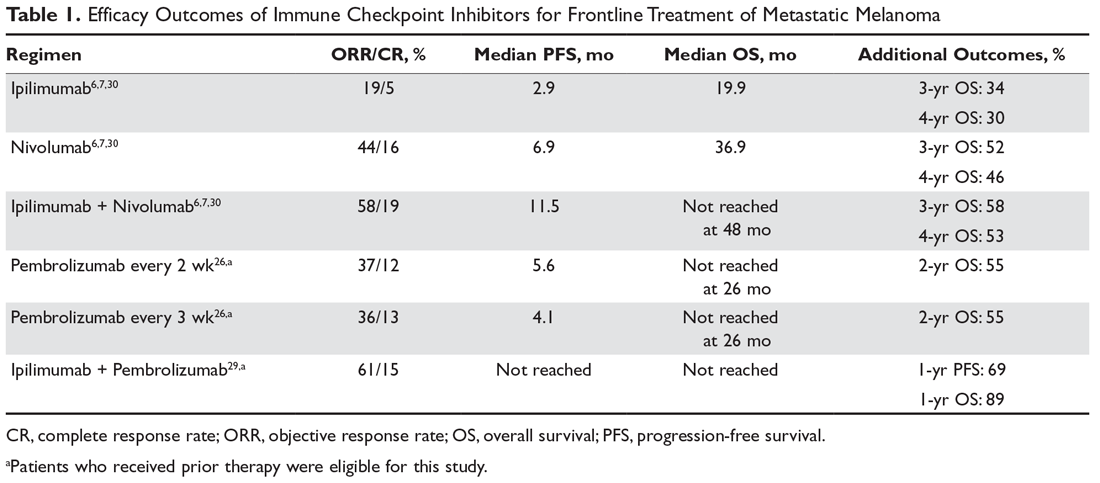
Despite the potential for durable responses, the majority of patients fail to respond to single-agent PD-1 therapy. Given that preclinical data had suggested the potential for synergy between dual inhibition of CTLA-4 and PD-1, clinical trials were designed to test this approach. The first randomized phase 2 trial that established superior efficacy with combination therapy was the CheckMate 069 trial comparing nivolumab plus ipilimumab to ipilimumab monotherapy. Combination therapy resulted in increased ORR (59% vs 11%), median PFS (not reached vs 3.0 months), 2-year PFS (51.3% vs 12.0%), and 2-year OS (63.8% vs 53.6%).28 Similarly, a phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and 1-year OS of 89%.29
The landmark phase 3 CheckMate 067 trial analyzed efficacy outcomes for 3 different treatment regimens including nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy in previously untreated patients with unresectable stage III or IV melanoma. The trial was powered to compare survival outcomes for both the combination therapy arm against ipilimumab and the nivolumab monotherapy arm against ipilimumab, but not to compare combination therapy to nivolumab monotherapy. The initial analysis demonstrated a median PFS of 11.5 months with combination therapy versus 6.9 months with nivolumab and 2.9 months with ipilimumab, as well as an ORR of 58% versus 44% and 19%, respectively (Table 1).6 The updated 3-year survival outcomes from CheckMate 067 were notable for superior median OS with combination therapy (not reached in combination vs 37.6 months for nivolumab vs 19.9 months ipilimumab), improved 3-year OS (58% vs 52% vs 34%), and improved 3-year PFS (39% vs 32% vs 10%).7 In the reported 4-year survival outcomes, median OS was not reached in the combination therapy group, and was 36.9 months in the nivolumab monotherapy group and 19.9 months in the ipilimumab monotherapy group. Rates of grade 3 or 4 adverse events were significantly higher in the combination therapy group, at 59% compared to 22% with nivolumab monotherapy and 28% with ipilimumab alone.30 The 3- and 4-year OS outcomes (58% and 54%, respectively) with combination therapy were the highest seen in any phase 3 trial for treatment of advanced melanoma, supporting its use as the best approved first-line therapy in those who can tolerate the potential toxicity of combination therapy7,30 The conclusions from this landmark trial were that both combination therapy and nivolumab monotherapy resulted in statistically significant improvement in OS compared to ipilimumab.
Toxicity Associated with Immune Checkpoint Inhibitors
While immune checkpoint inhibitors have revolutionized the treatment of many solid tumor malignancies, this new class of cancer therapy has brought about a new type of toxicity for clinicians to be aware of, termed immune-related adverse events (irAEs). As immune checkpoint inhibitors amplify the immune response against malignancy, they also increase the likelihood that autoreactive T-cells persist and proliferate within the circulation. Therefore, these therapies can result in almost any type of autoimmune side effect. The most commonly reported irAEs in large clinical trials studying CTLA-4 and PD-1 inhibitors include rash/pruritus, diarrhea/colitis, hepatitis, endocrinopathies (thyroiditis, hypophysitis, adrenalitis), and pneumonitis. Other more rare toxicities include pancreatitis, autoimmune hematologic toxicities, cardiac toxicity (myocarditis, heart failure), and neurologic toxicities (neuropathies, myasthenia gravis-like syndrome, Guillain-Barré syndrome). It has been observed that PD-1 inhibitors have a lower incidence of irAEs than CTLA-4 inhibitors, and that the combined use of PD-1 and CTLA-4 inhibitors is associated with a greater incidence of irAEs compared to monotherapy with either agent.31 Toxicities associated with ipilimumab have been noted to be dose dependent.18 Generally, these toxicities are treated with immunosuppression in the form of glucocorticoids and are often reversible.31 There are several published guidelines that include algorithms for the management of irAEs by organizations such as the National Comprehensive Cancer Network.32
For example, previously untreated patients treated with ipilimumab plus dacarbazine as compared to dacarbazine plus placebo had greater grade 3 or 4 adverse events (56.3% vs 27.5%), and 77.7% of patients experiencing an irAE of any grade.18 In the CheckMate 066 trial comparing frontline nivolumab to dacarbazine, nivolumab had a lower rate of grade 3 or 4 toxicity (11.7% vs 17.6%) and irAEs were relatively infrequent, with diarrhea and elevated alanine aminotransferase level each being the most prominent irAE (affecting 1.0% of patients).25 In the KEYNOTE-006 trial, irAEs seen in more than 1% of patients treated with pembrolizumab included colitis, hepatitis, hypothyroidism, and hyperthyroidism, whereas those occurring in more than 1% of patients treated with ipilimumab included colitis and hypophysitis. Overall, there were lower rates of grade 3 to 5 toxicity with the 2 pembrolizumab doses compared to ipilimumab (13.3% pembrolizumab every 2 weeks vs 10.1% pembrolizumab every 3 weeks vs 19.9% ipilimumab).5 In the CheckMate 067 trial comparing nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy, rates of treatment-related adverse events of any grade were higher in the combination group (96% combination vs 86% nivolumab vs 86% ipilimumab), as were rates of grade 3 or 4 adverse events (59% vs 21% vs 28%, respectively). The irAE profile was similar to that demonstrated in prior studies: rash/pruritus were the most common, and diarrhea/colitis, elevated aminotransferases, and endocrinopathies were among the more common irAEs.7
Alternative dosing strategies have been investigated in an effort to preserve efficacy and minimize toxicity. A phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and a 1-year OS of 89%. This combination led to 45% of patients having a grade 3 or 4 adverse event, 60% having irAEs of any grade, and only 27% having grade 3 or 4 irAEs.29 The CheckMate 067 trial studied the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.6 The CheckMate 511 trial compared different combination dosing strategies (nivolumab 3 mg/kg + ipilimumab 1 mg/kg versus nivolumab 1 mg/kg + ipilimumab 3 mg/kg) to assess for safety benefit. In the results published in abstract form, the reduced ipilimumab dose (nivolumab 3 mg/kg + ipilimumab 1 mg/kg arm) resulted in significantly decreased grade 3 to 5 adverse events (33.9% vs 48.3%) without significant differences in ORR, PFS, or OS.33