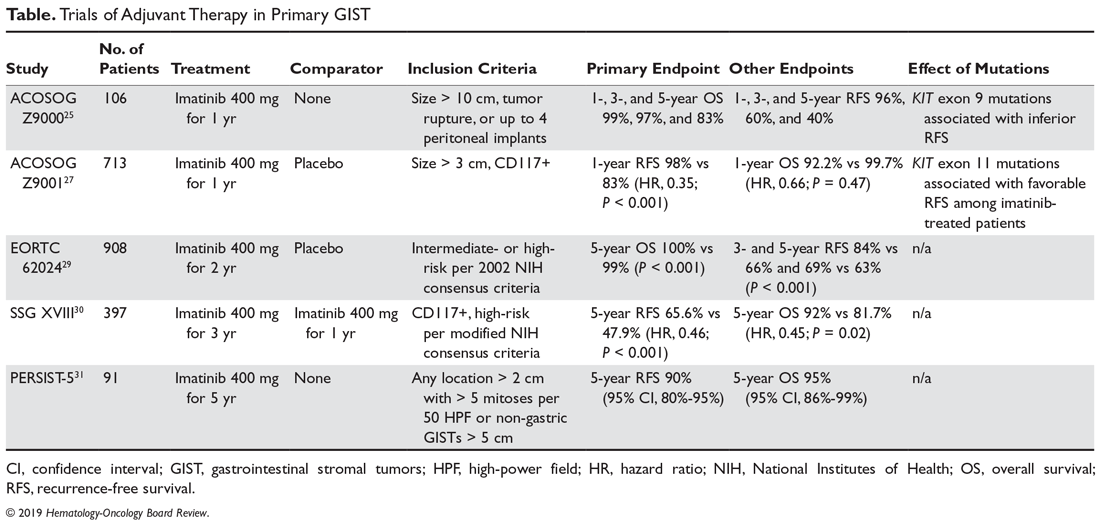
The Scandinavian Sarcoma Group (SSG XVIII) trial was a prospective randomized phase 3 trial that compared 3 years versus 1 year of adjuvant imatinib.30 Patients had to be enrolled within 12 weeks of the postoperative period and had to have GISTs that were CD117-positive and with a high estimated risk of recurrence, per the modified NIH consensus criteria (size > 10 cm, > 10 mitoses per 50 HPF, diameter > 5 cm with mitotic count > 5, or tumor rupture before or at surgery). Three years of adjuvant imatinib was associated with a 54% reduction in the hazard for recurrence at 5 years (65.6% vs 47.9%; HR, 0.46; P < 0.001) and a 55% reduction in the hazard for death at 5 years (OS 92% vs 81.7%; HR, 0.45; P = 0.02). Based on the results of this study, the FDA granted approval for the use of 3 years of adjuvant imatinib in patients with high-risk resected GISTs.
The observation that a longer duration of adjuvant imatinib was associated with superior RFS and OS led to studies to further explore longer durations of adjuvant imatinib. The PERSIST-5 (Postresection Evaluation of Recurrence-free Survival for Gastrointestinal Stromal Tumors With 5 Years of Adjuvant Imatinib) was a multicenter, single-arm, phase 2 prospective study of adjuvant imatinib with a primary endpoint of RFS after 5 years.31 Patients had to have an intermediate or high risk of recurrence, which included GISTs at any site > 2 cm with > 5 mitoses per 50 HPF or nongastric GISTs that were ≥ 5 cm. With 91 patients enrolled, the estimated 5-year RFS was 90% and the OS was 95%. Of note, about half of the patients stopped treatment early due to a variety of reasons, including patient choice or adverse events. Importantly, there were no recurrences in patients with imatinib-sensitive mutations while on therapy. We know that in patients at high risk of relapse, adjuvant imatinib delays recurrence and improves survival, but whether any patients are cured, or their survival curves are just shifted to the right, is unknown. Only longer follow-up of existing studies, and the results of newer trials utilizing longer durations of adjuvant treatment, will help to determine the real value of adjuvant therapy for GIST patients.32 Based on this study, it would be reasonable to discuss a longer duration of imatinib with patients deemed to be at very high risk of recurrence and who are tolerating therapy well. We are awaiting the data from the randomized phase 3 Scandinavian Sarcoma Group XII trial comparing 5 years versus 3 years of adjuvant imatinib therapy, and from the French ImadGIST trial of adjuvant imatinib for 3 versus 6 years. A summary of the aforementioned key adjuvant trials is shown in the Table.
When imatinib is commenced, careful monitoring for treatment toxicities and drug interactions should ensue in order to improve compliance. Dose density should be maintained if possible, as retrospective studies suggest suboptimal plasma levels are associated with a worse outcome.33
When should neoadjuvant imatinib be considered?
Neoadjuvant Imatinib
Neoadjuvant imatinib should be considered for patients requiring total gastrectomy, esophagectomy, or abdominoperineal resection of the rectum in order to reduce tumor size, limit subsequent surgical morbidity, mitigate tumor bleeding and rupture, and aid with organ preservation. Patients with rectal GISTs that may otherwise warrant an abdominoperineal resection should be offered a trial of imatinib in the neoadjuvant setting. There is no evidence for the use of any other TKI aside from imatinib in the neoadjuvant or adjuvant setting. With neoadjuvant imatinib, it is difficult to accurately assess the mitotic rate in the resected tumor specimen.
The RTOG 0132/ACRIN 6665 trial was a prospective phase 2 study evaluating the efficacy of imatinib 600 mg daily in the perioperative setting.34 The trial enrolled 50 patients, 30 with primary GISTs (group A) and 22 with recurrent metastatic GISTs (group B). Based on data from the metastatic setting revealing a time to treatment response of about 2.5 months, patients were treated with 8 to 12 weeks of preoperative imatinib followed by 2 years of adjuvant imatinib. Imatinib was stopped 24 hours preoperatively and resumed as soon as possible postoperatively. In group A, 7% of patients achieved a partial response (PR), 83% achieved stable disease, and 2-year progression-free survival (PFS) and OS were 83% and 93%, respectively. In group B, 4.5% of patients achieved a PR, 91% achieved stable disease, and 4.5% experienced progressive disease in the preoperative period; the 2-year PFS and OS were 77% and 91%, respectively. The results of this trial demonstrated the feasibility of using perioperative imatinib with minimal effects on surgical outcomes and set the rationale to use neoadjuvant imatinib in select patients with borderline resectable or rectal GISTs. Another EORTC pooled analysis from 10 sarcoma centers revealed that after a median of 10 months of neoadjuvant imatinib, 83.2% of patients achieved an R0 resection and only 1% progressed during treatment.35 After a median follow-up of 46 months, the 5-year disease-free survival and OS were 65% and 87%, respectively.