Owing to digital advances, we’re experiencing a reimagination of health care delivery. Consumers are now empowered to take more control of their own health information to make better informed decisions about their medical care and healthy living. These advances enable better health outcomes for patients.
This opportunity is supported by a new technological paradigm of digital health tools, like apps, that enable consumers to have more active engagement and access to real-time information about their health and activities. These tools allow consumers and providers to supersede the traditional, physical constraints of health care delivery and make the most of the opportunities offered by mobile technology.
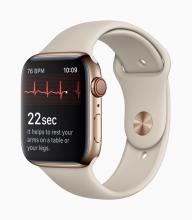
With these advances has come a new swath of companies that are investing in these new opportunities. These firms may be new to health care products and may not be accustomed to navigating the regulatory landscape that has traditionally surrounded these areas. A great example is the announcement of two mobile medical apps designed by Apple to work on the Apple Watch. One app creates an electrocardiogram, similar to traditional electrocardiograms, to detect the presence of atrial fibrillation and regular heart rhythm, while the other app analyzes pulse rate data to identify irregular heart rhythms suggestive of atrial fibrillation and notify the user. The FDA [Food and Drug Administration] worked closely with the company as they developed and tested these software products, which may help millions of users identify health concerns more quickly. Health care products on ubiquitous devices, like smartwatches, may help users seek treatment earlier and will truly empower them with more information about their health.
In the last few years, the FDA has been taking steps to encourage more development and greater innovation in the digital health space. With the launch of our Digital Health Innovation Action Plan last summer, we committed to implementing policies, adding expertise, and exploring a software precertification pilot program to bring clarity and efficiency to how we regulate digital health products.
This commitment is not only reflected in actions like approving or clearing new apps and launching our Digital Health Innovation Action Plan but also in what we hope to do in the future. That’s why in the FDA’s Fiscal Year 2019 Budget, we proposed to create a Center of Excellence for Digital Health that would advance modernizing our regulatory approach to help this industry grow and reach its full potential, while protecting patients.
Dr. Gottlieb is commissioner of the FDA and Dr. Shuren in director of the FDA Center for Devices and Radiological Health. Their comments are excerpted from an FDA statement released Sept. 12, 2018.