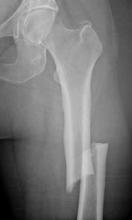
Long-term treatment with bisphosphonates may be related to an increased risk of atypical femur fractures in patients, who may experience weeks or even months of pain before fracture diagnosis, according to the final report issued by a task force convened by the American Society of Bone and Mineral Research.
The society convened the international, multidisciplinary task force to address reports of “atypical fractures of the subtrochanteric region of the hip and the femoral shaft” in patients on long-term bisphosphonate therapy. A statement issued by the society on Sept. 14 announcing the release of the report referred to it as “the most comprehensive scientific report to date” on this issue. The task force based its report on findings from a review of published and unpublished data, interviews with scientists at companies that manufacture these drugs, and Food and Drug Administration data.
The review included a review of 310 published case reports of patients (aged 36-92 years) with atypical subtrochanteric and femoral shaft fractures. Of these patients, 291 (94%) had been treated with bisphosphonates, mostly for osteoporosis. The median duration of treatment was 7 years, and most were treated for more than 5 years, according to the report, which points out that these fractures are rare, accounting for less than 1% of the overall rate of hip and femur fractures (J. Bone Miner. Res. 2010 Sept. 14 [doi:10.1002/jbmr.253]).
More than half of the patients with such femur fractures had experienced a prodrome of pain in the groin or thigh for weeks or months, and fractures were bilateral in more than 25% of the patients.
“We are concerned that there may be a relationship between these fractures and long-term bisphosphonate use and, although the risk is low, we want to make sure that people know about the warning signs,” the task force cochair and lead author of the report, Dr. Elizabeth Shane, said in the ASBMR statement. “Health professionals should know the warning signs of atypical femur fractures, and regularly ask patients on these drugs about groin or thigh pain,” added Dr. Shane, professor of medicine at Columbia University in New York.
She also recommended that every year, clinicians should evaluate whether bisphosphonate therapy is appropriate for patient on these drugs, and that that the drugs “should be reserved” for patients with Paget’s disease, patients with osteoporosis who are at high risk of fractures, and patients with certain cancers, she added.
In the statement, Dr. Shane also said that for the “vast majority of patients with osteoporosis, these drugs are an important weapon against fractures and their benefits far outweigh the risks of using them.”
The ASBMR task force recommendations include establishing an international registry of patients with these fractures, increasing research to determine whether bisphosphonates are the cause, and improving the labeling of these products so that health care professionals and patients are more aware of the potential for these fractures and the associated symptoms.
A statement issued by the FDA on Sept. 14 said that the agency “recommends that healthcare professionals be aware of the possible risk of unusual femur fractures in patients taking bisphosphonates. Patients should talk to their healthcare professional if they develop new thigh or groin pain so that they may be evaluated to rule out a femur fracture.”
These fractures remain rare. Findings from one study show that they account for 0.6 of every 1,000 fractures (CMAJ 2010;182:384-5).
Dr. Shane and other members of the task force disclosed relationships with manufacturers of bisphosphonates. Serious adverse events associated with bisphosphonates should be reported to the FDA’s MedWatch program.