CHICAGO – IDegLira, a fixed-ratio combination of insulin degludec and the glucagonlike peptide-1 agonist liraglutide, markedly outperformed each medication alone in a pivotal phase III clinical trial in patients with type 2 diabetes.
IDegLira combines the effects of insulin degludec and liraglutide in one daily injection, resulting in a substantial overall improvement in glycemic control with a low risk of hypoglycemia, weight gain, and GI adverse events," Dr. John B. Buse said in presenting the results of the phase III DUAL I (Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes) study at the annual scientific sessions of the American Diabetes Association.
The therapeutic rationale for the combination lies in the complementary characteristics of the two components. The net result is enhanced efficacy along with partial cross-cancellation of each drug’s side effects.
"Basal insulin is arguably the most powerful approach for controlling fasting blood glucose, and GLP-1 agonists have potent postprandial effects. The weight reduction associated with GLP-1 agonists could help mitigate the weight gain associated with basal insulin. The effects on alpha-cell function of GLP-agonists might reduce the hypoglycemia risk associated with basal insulin. And lastly, the process of individualized dosing that we use with basal insulin – getting the dose just right with regard to efficacy and safety – those concepts might reduce the GI side effects that are common with GLP-1 agonists," explained Dr. Buse, professor of medicine, chief of the division of endocrinology and metabolism, and executive associate dean for clinical research at the University of North Carolina, Chapel Hill.
All of these hypothesized benefits actually came to pass in DUAL I, a 26-week, international, open-label, phase III trial involving 1,663 type 2 diabetic subjects, all insulin-naive and inadequately controlled on metformin with or without pioglitazone. Eighty-three percent of patients were on metformin alone at enrollment, with the remainder on dual oral therapy.
Participants remained on their previous oral antidiabetic regimen during the 26-week study. They were randomized 2:1:1 to IDegLira, insulin degludec, or liraglutide (Victoza). Patients in the two insulin arms were started on 10 U/day of insulin degludec and titrated twice weekly in 2-unit increments, based upon their mean fasting plasma glucose. Ten units of IDegLira contain that amount of insulin degludec plus 0.36 mg of liraglutide. The maximum permitted dose of IDegLira contained 50 U of insulin degludec and 1.8 mg of liraglutide.
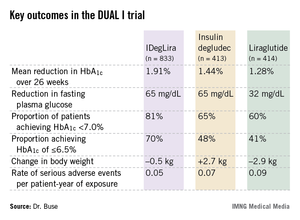
The primary study endpoint was reduction in hemoglobin A1c over the course of 26 weeks. Patients on IDegLira averaged a 1.91% drop to an HbA1c of 6.4%, significantly better than the 1.44% drop to 6.9% with insulin degludec and the 1.28% reduction to an HbA1c of 7.0% with liraglutide. In addition, the IDegLira group fared significantly better in terms of numerous secondary endpoints.
Particularly noteworthy, Dr. Buse continued, was the fact that fasting plasma glucose was reduced by an identical 65 mg/dL from a baseline of 165 mg/dL in the two-insulin treatment groups, even though the final mean daily dose of insulin degludec in the IDegLira group was 38 U, or 15 U/day less than in the group on insulin degludec alone. Confirmed hypoglycemic events occurred at a rate of 1.2 events over 26 weeks in the insulin degludec group and at a 32% lower rate in patients on IDegLira.
"That’s stunning in light of the fact that there was a 0.5% greater reduction in [HbA1c] in the IDegLira group," Dr. Buse observed.
Nausea was an issue for patients assigned to liraglutide. Roughly 10% of them per week reported nausea during titration, a rate that eventually fell off to about 3% per week after 14 weeks. In contrast, the proportion of patients in the IDegLira group who reported any nausea at all during the study was less than half that in the liraglutide group.
"This is likely related more to the titration program and the lower starting dose in the IDegLira group than to the final total milligrams of liraglutide," according to Dr. Buse. He noted that the final daily dose of liraglutide in the IDegLira group averaged 1.4 mg, compared with 1.8 mg in patients randomized to GLP-1 agonist monotherapy.
Earlier this year, Novo Nordisk, which is developing IDegLira, filed for marketing approval for the investigational agent from the European Medicines Agency. The company plans to file with the Food and Drug Administration later this year. However, the approval for insulin degludec was stalled after the FDA requested more cardiovascular safety data from the company in February 2013.
Session chair Julio Rosenstock of the Dallas Diabetes and Endocrine Center commented that the fixed-ratio combination is "a great concept" and hailed the phase III findings as "very, very impressive results." He wished, however, that the study had been set up blinded rather than open label.