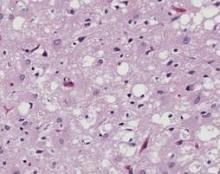
Two minimally invasive assays for detecting prions that are diagnostic of Creutzfeldt-Jakob disease in living patients have shown promise in preliminary studies reported by separate research groups Aug. 6 in the New England Journal of Medicine.
One assay tests epithelial samples obtained from nasal brushings and the other tests urine samples; both can be used in patients suspected of having the sporadic, inherited, or acquired forms of Creutzfeldt-Jakob disease (CJD), such as variant CJD and iatrogenic CJD. Both assays achieved sensitivities and specificities of 93%-100% in very small patient populations in these exploratory studies, which is better than the diagnostic accuracy of cerebrospinal fluid (CSF) testing.
If these findings are replicated in larger studies, both assays have the potential for establishing a definitive diagnosis of CJD in clinical settings. The test that uses nasal brushings may do so earlier in the course of the disease than has been possible previously, at least allowing the possibility of intervention for this invariably fatal neurodegenerative disorder.
In addition, the incidental finding that simple brushing of the olfactory mucosa yields an even higher quantity of prion "seeds" than are found in patients’ CSF suggests that infectivity may be present in the nasal cavity, which has important biosafety implications, the researchers noted.
In the first report, investigators applied real-time quaking-induced conversion (RT-QuIC) technology to olfactory epithelium samples from 31 patients who had rapidly progressive dementia and were referred for evaluation of possible or probable CJD from clinicians across Italy. These patients also underwent CSF sampling at the same time. A total of 12 patients with other neurodegenerative disorders (chiefly Alzheimer’s disease or Parkinson’s disease) and 31 patients at an ear, nose, and throat clinic who had no neurologic disorders served as controls, said Christina D. Orrú, Ph.D., of the Laboratory of Persistent Viral Diseases at the National Institute of Allergy and Infectious Diseases’ Rocky Mountain Laboratories in Hamilton, Mont., and her associates.
Obtaining the nasal brushings was described as a gentle procedure in which unsedated patients were first given a local vasoconstrictor applied with a nasal tampon, and then had a fiberoptic rhinoscope with a disposable sheath inserted into the nasal cavity to locate the olfactory mucosal lining of the nasal vault. A sterile, disposable brush was inserted alongside the rhinoscope, gently rolled on the mucosal surface, withdrawn, and immersed in saline solution in a centrifuge tube for further preparation.
The assays using this material yielded positive results for 15 of the 15 patients who had definite sporadic CJD, 13 of the 14 who had probable sporadic CJD, and 2 of the 2 patients who had inherited CJD. In contrast, all 43 control subjects had negative results. This represents a sensitivity of 97% (95% confidence interval [CI], 82-100) and a specificity of 100% (95% CI, 90-100) in this study population. In comparison, testing of CSF samples from the same patients only achieved a 77% sensitivity (95% CI, 57-89), Dr. Orrú and her associates said (N. Engl. J. Med. 2014 Aug. 6 [doi:10.1056/NEJMoa1315200]).
In addition, the "substantial" prion seeding found in the olfactory mucosa – greater than that in the CSF – raises the possibility that CJD prions could contaminate patients’ nasal discharges. "Nasal and aerosol-borne transmission of prion diseases have been documented in animal models, but there is no epidemiologic evidence for aerosol-borne transmission of sporadic CJD" to date, the investigators wrote.
It also is possible that medical instruments that come into contact with the nasal mucosa may become contaminated with prions, "which poses the question of whether iatrogenic transmission is possible. Therefore, further study of possible biohazards ... is warranted," they added.
In the second report, Fabio Moda, Ph.D., of the Mitchell Center for Research in Alzheimer’s Disease and Related Brain Disorders at the University of Texas, Houston, and his associates assayed urine samples using an extensive amplification technology for the presence of minute quantities of the misfolded prion protein in 68 patients with sporadic CJD, 14 with variant CJD, and 156 controls. The control group included 4 patients with genetic prion diseases, 50 with other neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, motor neuron disease, and progressive supranuclear palsy), 50 patients with nondegenerative neurologic disorders (chiefly cerebrovascular disease, multiple sclerosis, epilepsy, brain tumors, autoimmune encephalitis, and meningitis), and 52 healthy adults.
This assay achieved a sensitivity of 93% (95% CI, 66.1-99.8) and a specificity of 100% (95% CI, 98.4-100.0) in distinguishing CJD from other brain disorders and from brain health in this patient population, they said (N. Engl. J. Med. 2014 Aug. 6 [doi:10.1056/NEJMoa1404401]).