The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).
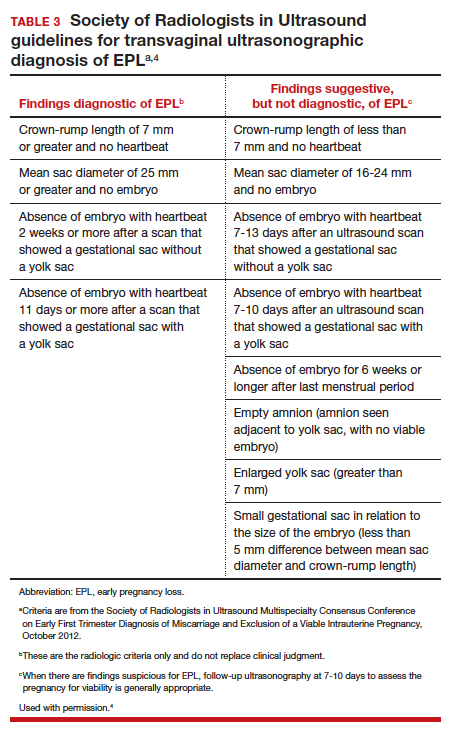
Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.
Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
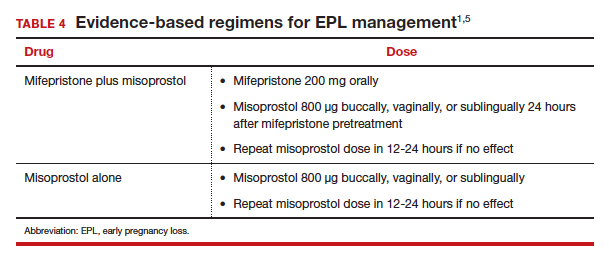
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.
Continue to: Uterine aspiration...