Vessel-sealing devices and hemostatic adjuvants are expanding the surgical armamentarium. These products provide a spectrum of alternatives that can serve you and your surgical patient well when traditional techniques for obtaining hemostasis fail to provide a satisfactory result. (Keep in mind, however, that technology is no substitute for excellent technique!)
In this article, we highlight three common scenarios in which topical hemostatic agents may be useful during gynecologic surgery. In addition, in the sidebar, five surgeons describe the hemostatic products they rely on most often—and tell why.
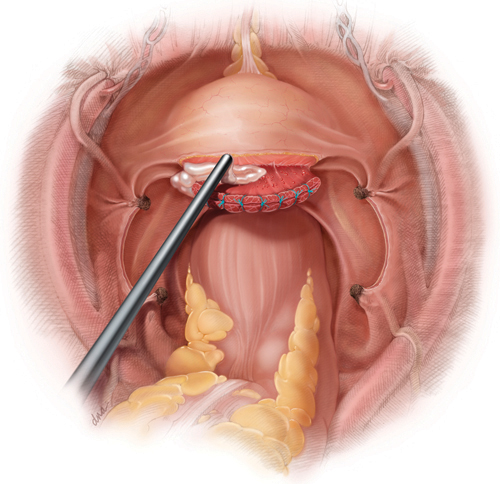
Following hysterectomy, persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to the site of bladder mobilization, may be managed with the aid of a topical hemostatic agent—in this case, a fibrin sealant.
When the site of bleeding is difficult to reach
CASE 1: Oozing at the site of bladder mobilization
You perform total hysterectomy in a 44-year-old woman who has uterine fibroids. After the procedure, you notice persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to where the bladder was mobilized.
How do you manage the oozing?
Wide mobilization of the bladder is a vital step in the safe performance of hysterectomy. Adhesions may complicate the process if the patient has had previous abdominal surgery, infection, or inflammation. Following mobilization of the bladder and removal of the uterus, bleeding may be visible along the adventitia of the posterior bladder wall or along the anterior surface of the vagina, distal to the cuff, as it is in this case (see the illustration).
Judicious application of an energy source is an option, but thermal injury to the bladder is a concern. A good alternative is proper placement of a hemostatic suture, but it can sometimes be difficult to avoid incorporating the bladder or injuring or obstructing the nearby ureter.
In this case, the location of the bleeding deep in the operative field poses a challenge, because of limited exposure and the proximity of the bladder and ureters. Virtually any hemostatic agent would work well in this circumstance (TABLE). For example, a flowable agent or fibrin sealant could be thoroughly applied to the area during a minimally invasive or open procedure and would naturally conform to the irregularities in the tissue, particularly the junction between the vagina and bladder flap.
A pliable product such as Surgicel Nu-Knit or Fibrillar would also work well in these circumstances, although successful application during laparoscopy may depend on the size of the trocar. For example, Nu-Knit would require trimming to a size suitable for passage through a trocar, made easier by moistening with saline. The weave of Fibrillar makes it more challenging to pass, intact, through a trocar; rolling the material into a cylindrical shape may reduce its diameter and allow it to pass more easily.
CASE 1: Resolved
You apply a fibrin sealant to the site of bleeding, and the oozing abates. Once complete hemostasis is ensured, you conclude the surgery and transfer the patient to recovery, where she does well.
Profiles in hemostasis: Strengths and weaknesses of topical agent
| Agent (brands) | Composition | Forms available | Mechanism of action | Advantages | Caveats | Duration | Relative cost* |
|---|---|---|---|---|---|---|---|
| Physical agents | |||||||
| Gelatin matrix (Gelfoam, Gelfilm, Surgifoam) | Porcine- derived collagen | Sponge, film, powder | Provides physical matrix for clot formation | Non-antigenic; neutral pH; may be used with thrombin | Material expansion may cause compression; Not for use in closed spaces or near nerve structures | 4–6 weeks | $ |
| Oxidized regenerated cellulose (Surgicel Fibrillar, Surgicel Nu-Knit) | Wood pulp | Mesh or packed fibers | Provides physical matrix for clot formation; acidic pH causes hemolysis and local clot formation | Pliable, easy to place through laparoscope; acidic pH has antimicrobial effect | Works best in a dry field. Acidic pH inactivates biologic agents, such as thrombin, and may increase inflammation. Avoid using excess material. | 2–4 weeks | $ |
| Microfibrillar collagen (Avitene, Instat, Helitene Helistat) | Bovine-derived collagen | Powder, non-woven sheet, sponge | Absorbable acid salt. Provides physical scaffold for platelet activation and clot initiation. | Sheet form may be passed through laparoscope; minimal expansion | Rare allergic reactions reported; may contribute to granuloma formation | 8–12 weeks | $$ |
| Biologically active agents | |||||||
| Topical thrombin (Thrombin-JMI, Recothrom, Evithrom, rh Thrombin) | Bovine, human, or recombinant | Liquid | Promotes conversion of fibrinogen to fibrin | May be combined effectively with physical agents of neutral pH; recombinant human thrombin will be available in the near future | Risk of blood-borne infection with non-recombinant human thrombin; risk of anaphylaxis and antibody formation with bovine thrombin | N/A | $$ |
| Hemostatic matrix (Floseal, Surgiflo) | Thrombin plus gelatin | Foam | Gelatin granules provide expansion and compression while thrombin initiates clot formation | May be used in areas of small arterial bleeding | Requires contact with blood | 6–8 weeks | $$$ |
| Fibrin sealants (Evicel, Tisseel, Crosseal) | Human | Liquid | Combination of fibrinogen and thrombin causes cleavage of fibrinogen to fibrin and resultant clot initiation | Fast-acting; hemostatic and adhesive properties; works well for diffusely oozing surfaces | Contraindicated in patients who have a history of anaphylactic reaction to serum-derived products or IgA deficiency | 10–14 days | $$$ |
| * Median cost for use in one case Key: $=inexpensive; $$=moderately expensive; $$$=expensive | |||||||



