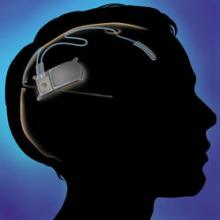
An implantable neurostimulator has been approved as a treatment for people with medically intractable epilepsy, based on a 3-month study of 191 patients, the Food and Drug Administration announced on Nov. 14.
The RNS stimulator, manufactured by Neuropace, is implanted in the cranium and is connected to 1-2 leads implanted near the seizure focus. It is designed "to detect abnormal electrical activity in the brain and respond by delivering imperceptible levels of electrical stimulation to normalize brain activity before an individual experiences seizures," according to Neuropace.
In the study of 191 patients, the number of seizures per month dropped by an average of 38% when the device was turned on, compared with an average 17% drop when the device was turned off, the FDA said in the statement announcing the approval. At the end of 3 months, seizures had been reduced by a median of 34% when the device was turned on, compared with a median 19% reduction when it was turned off. During a 2-year follow-up unblinded phase of the study, "data demonstrated a persistent reduction in seizure frequency," according to the FDA announcement.
The most common adverse effects of treatment were infections at the implant site and premature battery depletion, the agency said.
Patients who have RNS stimulators should not undergo magnetic resonance imaging, diathermy procedures, electroconvulsive therapy, or transcranial magnetic stimulation, which can cause permanent brain damage, even when the device is turned off, the FDA statement said.