News
Priorities for determining the etiology of incontinence
Letters from readers
Marjorie L. Pilkinton, MD; Dara Shalom, MD; and Harvey A. Winkler, MD



Dr. Winkler reports that he is a consultant to Astora Women’s Health, Boston Scientific, and Kimberly-Clark. Drs. Pilkinton and Shalom report no financial relationships relevant to this article.

First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Letters from readers
These surgeons review indications and demonstrate patient positioning and their minimally invasive fascia lata harvest technique.

Yes: Median episiotomy was associated with an increased rate of 3rd- and 4th-degree perineal laceration in both nulliparous and multiparous women...
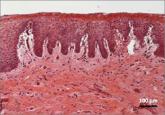
With more than 1 billion menopausal women likely to be affected by vulvovaginal atrophy worldwide by 2025, the need for effective remedies is...
No, provided the patient undergoes careful office evaluation instead, according to this systematic review and meta-analysis Rachaneni S, Latthe P...
