News
Priorities for determining the etiology of incontinence
Letters from readers
Marjorie L. Pilkinton, MD; Dara Shalom, MD; and Harvey A. Winkler, MD



Dr. Winkler reports that he is a consultant to Astora Women’s Health, Boston Scientific, and Kimberly-Clark. Drs. Pilkinton and Shalom report no financial relationships relevant to this article.

Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Letters from readers
These surgeons review indications and demonstrate patient positioning and their minimally invasive fascia lata harvest technique.

Yes: Median episiotomy was associated with an increased rate of 3rd- and 4th-degree perineal laceration in both nulliparous and multiparous women...
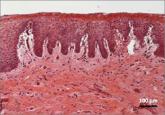
With more than 1 billion menopausal women likely to be affected by vulvovaginal atrophy worldwide by 2025, the need for effective remedies is...
No, provided the patient undergoes careful office evaluation instead, according to this systematic review and meta-analysis Rachaneni S, Latthe P...
