Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
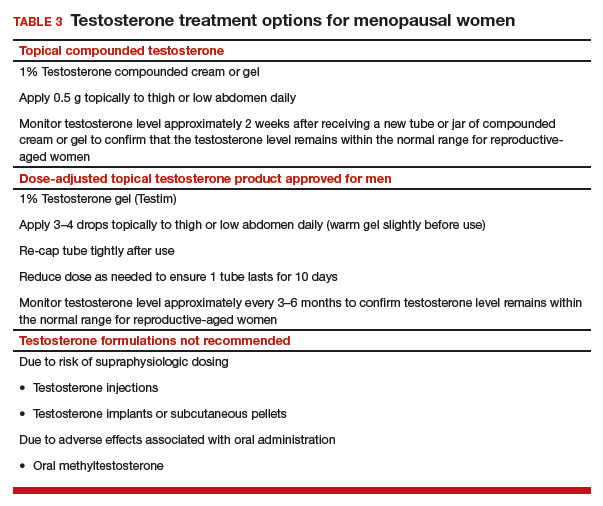
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.
Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.