Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
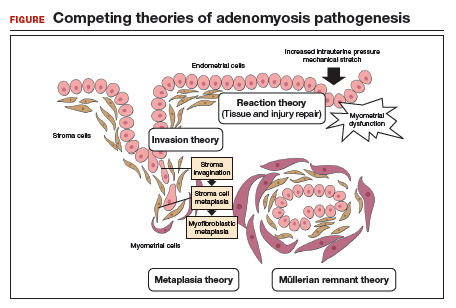
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…