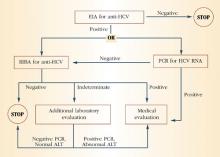
FIGURE 1 HCV infection testing algorithm
ALT=alanine aminotransferase; EIA=enzyme immunoassay; PCR=polymerase chain reaction; RIBA=recombinant immunoblast assay Source: Centers for Disease Control and Prevention
How is HCV transmitted?
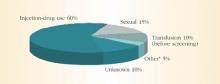
HCV spreads primarily through blood or fluids containing blood. Injection-drug use accounts for 60% of infections;1 a person who has used injection drugs for 5 years has a 60% to 90% chance of becoming infected with HCV. In fact, among injection-drug users, HCV infection is 4 times more common than HIV infection.8 The transfusion of blood or blood products accounts for another 10% of HCV cases. However, the transfusion-related risk has decreased markedly since the introduction of HCV screening in the blood banks in 1992. Figure 2 shows additional sources of HCV infection.
Although sexual transmission of HCV does occur, the virus is inefficiently spread in this manner. Evidence for this route of transmission has been accumulated from case-control and partner studies. Case-control studies have reported an association between HCV infection and exposure to a sex partner with a history of hepatitis or exposure to multiple sex partners.9,10 Cross-sectional studies suggest that the probability of HCV infection in the sexual partner of an HCV-positive patient is 0% to 3% in northern Europe or North America. Higher probabilities are found in southern Europe and the Far East.11 One prospective study showed no cases of sexual transmission of HCV from 94 HCV-positive females to their male part-ners.12 Another prospective study found a low incidence—12 infections per 1,000 per-son-years—among 449 sexual partners of HCV-positive individuals.13
Since the risk of spreading HCV through sexual contact is relatively low, individuals in long-term, stable relationships with an infected partner need not change their sexual practices. However, they may choose to use barrier methods to lower the chance of transmission even further. Individuals with high-risk sexual practices, such as having multiple sexual partners, should definitely use barrier methods.
FIGURE 2 Sources of infection for persons with hepatitis C
*Nosocomial, occupational, perinatal
Source: Centers for Disease Control and Prevention
What is the natural history of HCV infection?
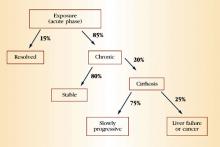
HCV accounts for approximately 20% of cases of acute hepatitis in the United States.14 The incubation period is approximately 6 weeks. Children and adults who acquire the infection usually are asymptomatic or have nonspecific symptoms of fatigue, malaise, anorexia, and weight loss. Some patients may present with jaundice. Serum aminotransferases can fluctuate during acute infection, and normalization occurs in 40% of individuals. However, this normalization does not necessarily represent a clearance of infection. Only 15% of patients clear the virus; the rest develop chronic infection.
Only 15% of patients clear the virus; the rest develop chronic infection.
During chronic infection, most patients are unaware that they are HCV-positive because, as with acute infection, they are asymptomatic or have mild, nonspecific symptoms such as fatigue. Again, serum aminotransferases typically fluctuate and are normal 30% of the time. Clinical progression to cirrhosis occurs in 20% of cases over a span of about 20 years. Once cirrhosis develops, the risk of decompensated cirrhosis or liver failure is about 3% to 4% per year. The annual risk of primary hepatocellular carcinoma in patients with cirrhosis is 1% to 4%. Data suggest that alcohol use is a predictive factor for progressive liver disease and liver cancer. Figure 3 summarizes the natural history of HCV infection.
FIGURE 3 Natural history of HCV infection
Is there a treatment for hepatitis C?
Over the past decade, there have been incremental improvements in hepatitis C therapy. The original mainstay of treatment was interferon alpha, with an initial response rate of 50% and a sustained response rate of only 10% to 15%.15-17 Later, combination therapy with interferon alpha and ribavirin raised the sustained response rate to 40%.18,19 However, both these therapies have substantial side effects and require frequent dosing schedules.
In January 2001, a new therapy, pegylated interferon alpha, was introduced. This modified form of traditional interferon alpha has fewer side effects and requires less frequent dosing. In addition, initial studies showed that it had a greater sustained-response rate than traditional interferon alpha alone.20 The Food and Drug Administration (FDA) recently approved it for the treatment of chronic hepatitis not previously treated with standard interferon alpha. A recent trial showed that the combination of pegylated interferon alpha with ribavirin had a sustained virologic response rate of 54%.21 None of these medications are approved for use in pregnancy or childhood.
To prevent progressive liver disease, patients chronically infected with HCV should avoid alcohol and other hepatotoxins and get vaccinated against hepatitis A and B. To reduce the risk of transmission to others, they should be advised not to donate blood and tissues; not to share toothbrushes, razors, or other personal-care items that may have blood on them; and to cover cuts and sores on the skin.