3 Eliminate postcoital testing
In most cases, postcoital testing should be abandoned.1 This recommendation is based on a good-quality trial that found that postcoital testing is unpredictive of cumulative pregnancy rates.8
4 Assess the uterine cavity
A thorough evaluation must include an appraisal of the uterine cavity, with the method tailored to the individual patient.1 A hysterosalpingogram with water-soluble dye is suggested. The patient should be instructed to use doxycycline for prophylaxis against pelvic infection prior to the hysterosalpingogram and advised to expect the test to be mildly to moderately uncomfortable. In some cases, pain can be severe, especially with abnormal findings.
Two benefits are expected with hysterosalpingography (HSG). One is a determination of whether the shape of the cavity appears normal, whether there is a suggestion of a congenital defect such as a double uterine cavity, or whether acquired abnormalities exist such as distortions of the cavity related to extrinsic compression from leiomyomata. The second is a transient boost to pregnancy chances by mechanical flushing of the tubes. Although hysteroscopy is the reference standard, sonohysterography using saline for visualization has the same diagnostic accuracy for detecting polyps or hyperplasia.9
5 Don’t neglect tubal factors
Assessment of tubal patency is another key element in the subfertility investigation.1 However, because each evaluation method has one or more technical limitations, a confirmatory test with a second method is recommended whenever results are abnormal. For example, HSG shows whether tubes are patent but not whether there are adhesions on the exterior of the tube. (Peritubal adhesions might compromise pick-up of the ovulated oocyte.)
The reference standard for assessing tubal patency is laparoscopy. In comparison, HSG has a sensitivity of 65% and a specificity of 83%. When it comes to evaluating peritubal adhesions, however, HSG is unreliable.10
HSG is used for both diagnosis and therapy. When it is used therapeutically, some experts believe the benefits of oil-based contrast media are superior to those of aqueous media. We at first were inclined to agree, since a Cochrane systematic review concluded that HSG with oil-based media enhanced pregnancy chances (compared with no HSG), and that chances of post-HSG pregnancy were greater with oil-based media (compared with aqueous media).11
However, most board-certified reproductive endocrinologists choose aqueous media, since it is safer, less expensive, and easier to use.12 In fact, roughly 90% of reproductive endocrinologists use aqueous media, according to a 1994 survey.12 In addition, a well-designed, prospective, multisite, randomized comparison of oil and aqueous media, with the power to detect a 10% difference, failed to confirm the superiority of oil-based media.13 This trial was not included in the Cochrane review.
Comparative studies suggest 2 alternative methods for assessing tubal health. In areas with a high prevalence of chlamydia, the measurement of serum antibodies to chlamydia is as accurate as HSG in diagnosing tubal disease.14 Contrast hysterosonography concurs with HSG in 70% of cases.15
6 Evaluate the peritoneum
When there is a strong suspicion of endometriosis or pelvic adhesions, laparoscopy is recommended, particularly if aggressive empirical therapy is planned that would entail greater than average risk or cost.1
Like HSG, laparoscopy is used both diagnostically and therapeutically. Although we consider it a prerequisite for diagnosing endometriosis, the ultrasonographic diagnosis of endometriomata is 90% accurate.16
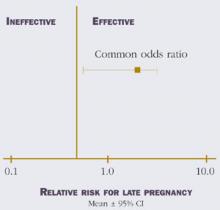
The surgical reduction of dark endometriotic lesions in women with minimal/mild endometriosis appears to be modestly effective (Figure 2).17-19
FIGURE 2 Efficacy of surgical reduction in minimal/mild endometriosis
7 Look for male defects
As we mentioned earlier, the male partner should be screened if no pregnancy has occurred after 1 year of unprotected intercourse.2 If risk factors exist—or the male partner is uncertain of his fertility—earlier evaluation may be justified. A reproductive history and 2 semen analyses comprise the initial evaluation.
Unfortunately, we lack “true” reference figures for semen parameters.2 Nevertheless, despite overlapping parameters between fertile and subfertile populations, subfertility should be suspected if the sperm concentration is less than 13.5×106/mL, if motility is less than 30%, or if normal morphology is less than 9% using strict criteria.20
Even so, the use of strict criteria to assess sperm morphology is not any more accurate than the criteria developed by the World Health Organization (WHO)21 when it comes to discriminating between fertile and subfertile men.22 Menkveld and colleagues used receiver operator characteristic (ROC) curves—which relate true-positive results to false-positive results—to study the feasibility of discriminating between fertile and subfertile men using semen parameters.22 WHO criteria had a marginally greater area under the curve than did strict criteria, with the 95% confidence intervals of the 2 sets of criteria overlapping. (Minimum values for in vivo conception are estimated as 20% normal morphology according to WHO criteria and 3% normal morphology according to strict criteria.22)