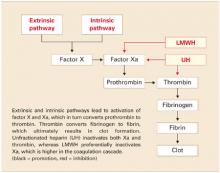
Question 3How does LMWH differ from unfractionated heparin?
LMWH is more efficient. Both UH and LMWH contain an essential pentasaccharide within their polymer structure that binds to and enhances antithrombin III, which in turn inhibits thrombin and activated factor X (Xa). Because of its smaller size, LMWH preferentially inhibits Xa, which is higher in the coagulation cascade. Inhibition of a single molecule of Xa prevents the formation of many molecules of thrombin. Molecule for molecule, LMWH is a more efficient anticoagulant than UH (FIGURE).11
The second way that LMWH differs from UH also relates to the molecule’s size. Smaller heparin molecules are less likely to be deactivated by tissue proteins. This results in improved bioavailability of the administered dose. Greater bioavailability translates to a more predictable dose-response relationship, a long half-life, and better anticoagulation.12
FIGURE Simplified schematic of the coagulation cascade
Question 4What are the clinical advantages of LMWH?
LMWH has longer-lasting effects and subcutaneous dosing. It also has fewer side effects than UH.
Because of its large size and positive charge, UH has an unfavorable pharmacokinetic profile. Tissue proteins interfere with and deactivate it, and many of these proteins increase in pregnancy and with advancing gestation. Heparin tissue levels are therefore erratic and unpredictable and often lead to periods of subtherapeutic coverage. This is true even with intravenous (IV) dosing.
Rapid absorption, no intravenous dosing. In contrast, because of its smaller size, LMWH is rapidly and predictably absorbed from a subcutaneous injection. Intravenous dosing is not necessary to obtain adequate tissue levels. Once in tissue, it is deactivated more slowly and therefore maintains its anticoagulation effect longer. Consequently, patients are exposed to fewer periods of subtherapeutic anticoagulation. A longer half-life also translates to more favorable dosing routes (subcutaneous rather than IV) and regimens (daily versus twice daily). Similarly, since the dose-response is predictable and tissue levels are more constant, frequent monitoring of treatment response is not routinely necessary.
Fewer side effects. Another advantage of LMWH over UH is the improved side-effect profile. Patients on LMWH have decreased risk of hemorrhage, osteoporosis, and antibody-mediated thrombocytopenia.11 Although most data regarding these advantages come from the nonpregnant population, it is plausible to speculate that these traits also are present in pregnant women (TABLE 2).
TABLE 2
Advantages and disadvantages of LMWH in pregnancy
| ADVANTAGES |
| More effective anticoagulation |
| Better dose-response |
| Longer half-life |
| Better dosing route |
| Decreased need for monitoring |
| Fewer side effects |
| DISADVANTAGES |
| Longer half-life |
| Risk of hematoma with epidural anesthesia |
| Not fully reversible with protamine sulfate |
| Anticoagulation effect difficult to monitor |
| Higher cost |
Question 5What are the disadvantages?
They include the long half-life, risk of hematoma with epidural anesthesia, lower efficacy of the antidote, monitoring difficulty, and higher cost.
The long half-life of LMWH is both an advantage and disadvantage. For example, when UH is administered intravenously, it has a half-life of 30 to 60 minutes. When it is given subcutaneously, the half-life is 1 to 2 hours. This means that a patient can undergo vaginal delivery or even surgery within hours of her last subcutaneous UH injection.
In contrast, LMWH has a half-life of approximately 4 hours. A recent dose may increase the risk of operative morbidity in the form of overt or delayed hemorrhage, hematoma, or wound dehiscence.
Not for use with epidural anesthesia. Case reports of epidural hematomas after regional anesthesia during orthopedic procedures have caused considerable concern about the use of LMWH and the placement of a neuraxial block such as a spinal or epidural.13 Many anesthesiologists will not place an epidural or spinal within 24 hours of a LMWH dose.14 However, as experience with these agents in the nonpregnant population expands, a more evidence-based approach is likely to develop.
Antidote less effective. Protamine sulfate is a strong base that binds with the positively charged UH molecule, thereby serving as an antidote through competitive inhibition. Because of its smaller size, LMWH is reversed by protamine to a lesser degree (approximately 60% effective).14 Therefore, hemorrhage associated with LMWH may require replacement of blood components, which carries the risks of infection and transfusion reactions.
Difficult to monitor. The anticoagulation effect of UH can be reliably monitored by the activated partial thromboplastin time (aPTT), which is a widely available test with a rapid turnaround. However, the anticoagulation effect of LMWH is not reflected by the aPTT. Assessment of LMWH activity requires assessment of the antifactor Xa level, a test that is not universally available and has a longer turnaround.
Higher cost. Another limitation of LMWH is its cost. A single, subcutaneous, prophylactic 40-mg dose of enoxaparin costs about $30, compared with about $1 for an equivalent dose of unfractionated heparin (5,000 U subcutaneously twice a day). Because of its increased cost, many insurance companies do not authorize the use of LMWH for prolonged periods such as pregnancy and the postpartum period. However, studies in the nonpregnant population have demonstrated overall decreased cost over UH due to the reduced need for monitoring, shorter length of hospital stay, and diminished treatment failure.