The long-term success of endometrial ablation devices as a whole depends on several conditions. Foremost, the entire class of devices should demonstrate efficacy on par with hysteroscopic ablation. Currently, efficacy ranges from 80% to 95% (short-term follow-up).11 The goal of minimally invasive procedures should be a sustainable 92% rate of amenorrhea, hypomenorrhea, or light, periodic menses. A long-term failure rate of 25% is unacceptable.22-24 If the devices can, by their simplicity, be adapted to more or less universal office application and attain a 5-year success rate of 90% or higher, they will become the standard of care.
One size does not really fit all
Serious complications from endometrial ablation devices occur with regular frequency and must be eliminated or greatly reduced. Perforation is a significant problem and may be related to the “one-size-fits-all” design of the device. Perhaps a range of sizes needs to be produced and fitted to the individual uterine cavity.
If such complications as perforation and burns to the bowel, cervix, vagina, and vulva can be eliminated or relegated to rarity, then a happy future for these procedures lies beyond the horizon.
Price ceiling should be set at $1,000
If an operation can consistently be performed for less than $1,000 total cost—the cost of in-hospital endometrial ablation—it will gain mass appeal. In hospitals and so-called surgicenters, ablations are expensive and, therefore, less attractive to self- or third-party payers. If fees are based on the volume of cases, then a procedure may be price-efficient.
Outcome depends on patient selection
Poorly screened patients who have underlying hyperplasia may develop postablation carcinoma. Women who have dysmenorrhea before the procedure can be predicted to suffer from it afterward. Older women (ie, 40 years or older) will have better long-term success than younger women. And women with a large uterus or myomas will have a higher failure rate than women with smaller cavities (ie, less than 10 cm in length).
What this means for the individual surgeon
Although minimally invasive techniques are relatively easy to perform and simple to learn, each part of the procedure requires careful application and great attention to detail. Perforation of the uterus and leakage of scalding hot liquid must be avoided. If these complications occur, prompt diagnosis and appropriate treatment are critical. The removal of these procedures from the operating room to the office as well as competitive pricing of instrumentation will make nonhysteroscopic, minimally invasive endometrial ablation more cost-effective.
The modern era of practical endometrial ablation began in 1981, when Goldrath and colleagues19 reported Nd-YAG laser photovaporization of the endometrium via hysteroscopy for treatment of excessive uterine bleeding. Two years later, DeCherney and Polan20 reported hysteroscopic control of abnormal uterine bleeding using the urologic resectoscope.
Over succeeding years, Baggish and Baltoyannis21 and Baggish and Sze22 reported extensive experience with hysteroscopic endometrial ablation in both high- and average-risk patients, including long-term follow-up of 568 cases over 11 years. Garry and colleagues23 reported a large series of 600 cases from the United Kingdom. Not only did these laser techniques prove to be effective, achieving amenorrhea rates ranging from 30% to 60%, but overall control of abnormal bleeding exceeded 90%. In the large series involving approximately 1,200 cases, no uterine perforations were reported.21-23 The major complication: Fluid overload secondary to vascular uptake of distension medium.
In Europe and the United Kingdom, most hysteroscopic treatment of abnormal bleeding involved endometrial resection using the cutting loop of the resectoscope. In the United States, ablation with the ball electrode of the resectoscope largely replaced the Nd-YAG laser because the resectoscopic trigger mechanism required less skill and hand–eye coordination than the hand–finger-controlled movement of the 600- to 1,000-micron laser fiber.24-26
A search for more benign techniques
A 1997 UK survey analyzed 10,686 cases of hysteroscopic endometrial destruction and identified 474 complications.27 Resection alone had a complication rate of 10.9% and an emergency hysterectomy rate of 13 for every 1,000 patients. Laser ablation had a complication rate of 5.5% and an emergency hysterectomy rate of 2 for every 1,000 patients, and the corresponding figures for rollerball ablation were a 4.5% complication rate and 3 emergency hysterectomies for every 1,000 patients. Two deaths occurred (in 10,000 cases) and were associated with loop excision.
Published data indicated that:
- Successful outcomes after endometrial ablation or resection were directly proportional to the skill of the surgeon
- Complications, particularly serious complications, were related to the experience and skill of the surgeon
- Infusion of uterine distension medium, particularly hypo-osmolar solutions, was associated with serious complications when fluid deficits exceeded approximately 500 to 1,000 mL.
As a result, a number of investigators sought to develop new surgical techniques to control abnormal uterine bleeding that would minimize the skill required by the surgeon (requiring only insertion of a cannula into the uterus and a “cookbook” ablation procedure), eliminate the need for distension medium and general anesthesia, and attain efficacy equivalent to earlier techniques.
A quartet of options
Among the devices that resulted were:
- A microwave technique, described by several investigators.8-10 Its chief drawback: High-frequency electrical leakage with the potential to cause thermal burns.
- An intrauterine balloon device distended with sterile water or saline is heated in situ to 85° to 90° Celsius, thereby cooking the endometrium.
- An electrode-bearing device that features an array of monopolar electrodes over the endometrium-facing aspect of a balloon or bipolar electrodes over a porous bag.
- Devices that circulate a small volume of hot saline freely within the uterine cavity. Hydrothermablation delivers 10 to 12 mL of preheated saline into the uterus under low pressure. A similar technique delivers 10 to 12 mL of cool water or saline into the uterus through a sealed cannula, followed by in situ heating and circulation of the fluid at low pressure via a computer-controlled device.
Safety studies were required by the FDA and were performed on all these devices, and the risk of complications appeared to be negligible.1,2 As this article illustrates, that is not the case.
Four devices, four ways of achieving ablation
Since the advent of nonhysteroscopic, minimally invasive endometrial ablation devices, four distinct techniques have gained widespread use
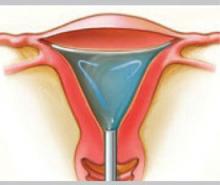
Hydrothermablation
The closed-loop system (HTA) ablates the lining of the endometrium under hysteroscopic visualization by recirculating heated saline within the uterus. The modified hysteroscope allows the operator to view the ablation as it occurs within the uterine cavity.
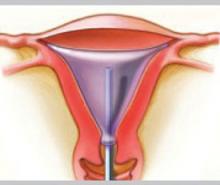
Balloon ablation
Balloon ablation (Thermachoice) features a double-dip balloon construction that conforms to the contours of the uterine cavity. The saline or water in the balloon is heated in situ. This device requires an undistorted uterine cavity, relies on the integrity of the balloon to prevent forward or retrograde spillage of scalding water, and is time-controlled.
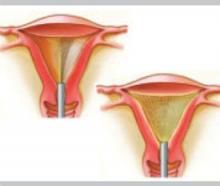
Radiofrequency technology
The three-dimensional gold-plated bipolar mesh electrode (NovaSure) is inserted into the uterine cavity and advanced toward the fundus. Once it is properly positioned (above, left), the system is activated to produce 180 W of bipolar power. A moisture-transport vacuum system draws the endometrium into contact with the mesh to enhance tissue vaporization and evacuate debris.
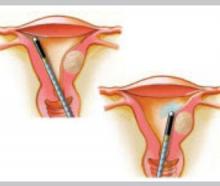
Microwave energy
Microwave energy is emitted from the tip of the device (Microsulis), which is moved back and forth in a sweeping manner, from the fundus to the lower uterine segment. The device directly heats tissue to a depth of 3 mm, with conductive heating of adjacent tissue for an additional 2 to 3 mm. The total 5- to 6-mm depth ensures coagulation and destruction of the basal layer. Microwave energy does not require direct contact with the tissue, as it will “fill the gap” caused by cornual and fibroid distortions.