Mr. C, age 31, who has a 7-year history of schizophrenia and is currently on perphenazine, 24 mg twice a day, presents for psychiatric admission after experiencing paranoid delusions. Notable symptoms include delusions of reference and persecution, along with affective flattening and intermittent suicidal ideation. Perphenazine is tapered, and he is started on quetiapine, titrated to 600 mg/d.
Past antipsychotic trials include aripiprazole, olanzapine, paliperidone, haloperidol,
and ziprasidone. Because of his refractory symptoms and tolerability issues with other antipsychotics, Mr. C is switched to clozapine, 400 mg/d. His symptoms improve, but he experiences dose-limiting sialorrhea. Risperidone, 1 mg/d, is added to clozapine, which helps his psychosis and improves his functional status. Additionally, Mr. C develops enough insight to recognize his delusions and use skills learned in psychotherapy to cope with them.
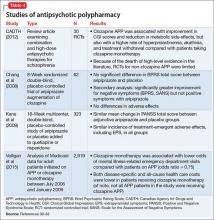
Antipsychotic polypharmacy (APP), the concurrent use of ≥2 antipsychotics, is a topic of debate among mental health care providers. Studies indicate the prevalence of APP can reach upwards of 40%, with 1 systematic review citing more recent median APP prevalence in North America as 17%, an increase from a median of 12.7% in the 1980s.1 Other studies cite more recent figures as around 20%.2,3
The literature lists several reasons for use of long-term APP, including:
- incomplete cross-titration
- accidental continuation of APP that was intended to be temporary
- monotherapy failure
- mitigation or enhancement of effects of other antipsychotics (Table 1).1,4
Other factors include direct-to-consumer advertising, external pressures to decrease hospital stays, and low doctor-to-patient ratios.5 Although it can take as long as 16 weeks to see clinically significant improvement with an antipsychotic, prescribers might expect results after 4 weeks of treatment.6 Therefore, treatments could be labeled ineffective because trials did not last long enough, leading to premature use of polypharmacy. Combinations of a first- and second-generation antipsychotic (SGA) or 2 SGAs are most common.2,7,8
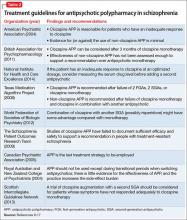
Treatment guidelines (Table 2)9-17 suggest APP could be considered after several failures of monotherapy, including clozapine monotherapy, although some guidelines do not address the issue or recommend against APP because of lack of efficacy and safety data. Additionally, APP poses safety concerns (Table 3).18-22 Recommendations for APP with combinations that do not include clozapine generally are not provided, because high-level evidence to support this strategy is lacking. Data on safety and efficacy of APP are mixed, with much of the literature dominated by case reports and uncontrolled studies.19
What to initiate
Clozapine. Higher-level evidence is available for clozapine APP. The combination of clozapine and risperidone is one of the most thoroughly studied and, therefore, is a reasonable first choice. Randomized controlled trials (RCTs) examining clozapine plus risperidone23-29 have yielded mixed results and have not provided conclusive information regarding benefit for positive vs negative symptoms.24-28
One RCT reported a significant change in Brief Psychiatric Rating Scale (BPRS) total and positive symptom scores.27 Other RCTs have shown a non-significant trend toward greater change in total, positive, and negative symptom scores with the clozapine-risperidone combination compared with clozapine monotherapy.25,28 In terms of cognition, this combination provided no additional benefit.23 Response, defined as ≥20% reduction in total BPRS or Positive and Negative Syndrome Scale (PANSS) scores, for clozapine plus risperidone range from 13% to 83%, compared with 8% to 29% for clozapine plus placebo.24,25,27,29
Data from 1 study27 suggest a number needed to treat of 4 to achieve at least a 20% improvement in BPRS scores with clozapine plus risperidone vs clozapine monotherapy. Across these studies, the average risperidone dosage was 4 mg/d, although using the lowest effective dosage is encouraged. A small number of RCTs and articles examining other APP combinations (Table 4)30-33 have yielded mixed results.
Overall, APP appears to be well-tolerated, although it is associated with an increased risk of adverse effects, including sedation, extrapyramidal symptoms, hyperprolactinemia, sexual dysfunction, cognitive impairment, anticholinergic effects, hyperlipidemia, and diabetes.23,24,34-36 Surprisingly, 1 literature review36 found no association between APP and increased risk of orthostasis. Increased occurrence of sedation, hyperprolactinemia, and an elevated fasting blood glucose level have been found for clozapine plus risperidone compared with clozapine monotherapy.24-26,28
Aripiprazole. Adjunctive aripiprazole, a dopamine partial agonist, could reduce elevated prolactin levels caused by other antipsychotics.32 In a study37 of 56 patients taking haloperidol who had hyperprolactinemia, prolactin levels normalized in 88.5% of patients taking adjunctive aripiprazole, 30 mg/d, compared with 3.6% of those with added placebo. Furthermore, results from 2 RCTs38,39 of patients taking clozapine or olanzapine suggest adjunctive aripiprazole could improve weight and metabolic profile. Therefore, adding aripiprazole to existing antipsychotic regimens is reasonable for patients with drug-induced symptomatic hyperprolactinemia or metabolic effects and who cannot be easily switched to another antipsychotic.