Compared with first-generation antipsychotics, second-generation antipsychotics (SGAs) have a lower risk for extrapyramidal symptoms. Yet tardive dyskinesia (TD) remains a concern because of the widespread use of SGAs for multiple indications.1 Prior to April 2017, clinicians had no FDA-approved TD treatment options. The most widely used agent worldwide, tetrabenazine, had positive efficacy data in TD trials over the past 45 years but was not available in the United States until 2008, and its sole indication was for chorea associated with Huntington’s disease.2 Moreover, the use of tetrabenazine involved slow titration, multiple daily dosing, cytochrome P450 (CYP) 2D6 genotyping for doses >50 mg/d, and tolerability issues.
Tetrabenazine is an inhibitor of vesicular monoamine transport type 2 (VMAT2), a transport protein located almost exclusively in the CNS whose role is to place monoamine neurotransmitters (dopamine, serotonin, norepinephrine) into presynaptic vesicles. By decreasing dopamine transport into these presynaptic vesicles, synaptic dopamine release is lessened, thus reducing postsynaptic dopamine D2 receptor activity and the severity of dyskinetic movements.1
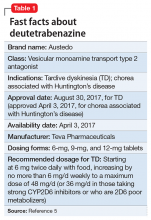
To overcome tetrabenazine’s kinetic limitations, adverse effect profile, and CYP2D6 monitoring requirements, 2 strategies were pursued, resulting in the availability of 2 novel VMAT2 inhibitors. Valbenazine, a molecule that is slowly converted to 1 of tetrabenazine’s active metabolites, was FDA-approved for TD on April 11, 2017 (see "Valbenazine for tardive dyskinesia"3). Deutetrabenazine is a version of tetrabenazine modified with the stable nontoxic isotope deuterium that exhibits improved kinetics and tolerability compared with tetrabenazine. Deutetrabenazine was approved for chorea associated with Huntington’s disease on April 3, 2017,4 and was subsequently approved for TD on August 30, 2017 (Table 1).5
In 2 pivotal 12-week clinical trials, deutetrabenazine significantly reduced TD severity as measured by Abnormal Involuntary Movement Scale (AIMS) scores (see Efficacy).6,7