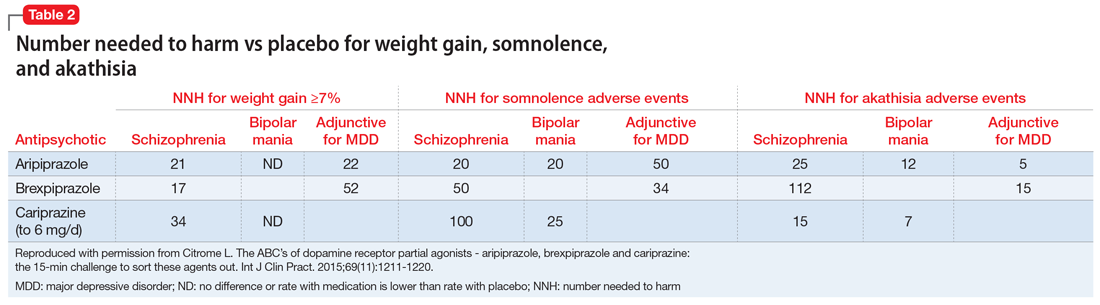
Tolerability. For all 3 agents, rates of discontinuation because of an adverse event were not higher for active medication vs placebo for the schizophrenia studies, suggesting excellent overall tolerability.2,19,25 For the other disease states, NNH values ranged from 17 (adjunctive use of aripiprazole for bipolar mania) to 100 (aripiprazole monotherapy for bipolar mania), representing reasonable overall tolerability. For the most commonly encountered adverse event for each medication, the NNH values ranged from 5 (akathisia for aripiprazole for adjunctive use in MDD) to 50 (increased weight for brexpiprazole for schizophrenia). Of special interest are the adverse events of weight gain ≥7% from baseline, somnolence adverse events, and akathisia adverse events; the NNH values vs placebo for these are listed in Table 21. Pragmatically, NNH values <10 are likely to be more clinically relevant. For aripiprazole, brexpiprazole, and cariprazine for the treatment of schizophrenia, none of the NNH values for weight gain, somnolence, or akathisia were <10; however, this was not the case for the mood disorders, where in general, akathisia was more frequently observed for each of the agents. For the indication of schizophrenia, the rank order for propensity for weight gain appears to be brexpiprazole > aripiprazole > cariprazine, the propensity for somnolence aripiprazole > brexpiprazole > cariprazine, and the propensity for akathisia cariprazine > aripiprazole > brexpiprazole; however, this is by indirect comparison, and appropriately designed head-to-head clinical trials will be necessary in order to accurately assess these potential differences.
Because of the partial agonist activity at the dopamine D2 receptor, aripiprazole, brexpiprazole, and cariprazine are less likely to cause hyperprolactinemia than other first-line first- or second-generation antipsychotics. Other differentiating features of the dopamine receptor partial agonists compared with other choices include a relative lack of effect on the QT interval.38 In general, as predicted by their relatively lower binding affinities to histamine H1 receptors, the dopamine receptor partial agonists are not especially sedating.39
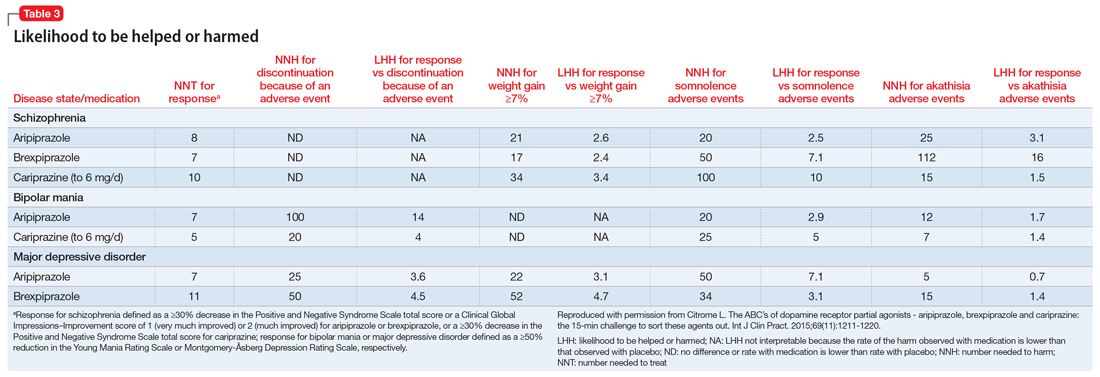
Likelihood to be helped or harmed
The concept of likelihood to be helped or harmed (LHH) can be useful to assess benefit vs risk, provided you select a relevant harm to contrast with the expected benefit.40 Table 31 provides the NNT for response, NNH for discontinuation because of an adverse event (where applicable), the NNHs for weight gain ≥7%, somnolence adverse events, and akathisia adverse events, together with the calculated LHH (where applicable). With the exception of aripiprazole for the treatment of MDD when comparing response vs akathisia, all LHH values are >1.0, and thus the benefit (response) would be encountered more often than the harm. When LHH values are ≥10, this can be interpreted that one would encounter a response at least 10 times more often than the adverse event of interest. This was observed for brexpiprazole for the treatment of schizophrenia when comparing response vs akathisia, for cariprazine for schizophrenia when comparing response vs somnolence, for aripiprazole for bipolar mania when comparing response vs discontinuation because of an adverse event, and for cariprazine for bipolar mania when comparing response vs somnolence.
Beyond acute studies
When treating patients with schizophrenia, delaying time to relapse is a main goal. In placebo-controlled randomized withdrawal studies of oral aripiprazole, brexpiprazole, and cariprazine in patients with schizophrenia, observed relapse rates vs placebo were reported, allowing the calculation of NNT vs placebo for the avoidance of relapse.41-44 These NNT values were similar and ranged from 4 to 5. For aripiprazole, relapse rates vs placebo in the 26-week study were 34% vs 57%, resulting in a NNT of 5 (95% CI 3 to 9); brexpiprazole, 52-week study, 13.5% vs 38.5%, NNT of 4 (95% CI 3 to 8); and cariprazine, 72-week study, 25% vs 47.5%, NNT of 5 (95% CI 3 to 11). In addition, cariprazine, 4.5 mg/d, has been directly compared with risperidone, 4 mg/d, in a 26-week double-blind study in non-geriatric adult patients with schizophrenia and predominant negative symptoms for at least 6 months.45 Cariprazine was superior to risperidone on the PANSS–Negative Factor Score, and response to treatment (decrease ≥20% in PANSS–Negative Factor Score) was achieved by more patients treated with cariprazine by 26 weeks than those treated with risperidone (69% vs 58%, NNT 9 [95% CI 5 to 44]).
Caveats
The harms discussed in this article are primarily from acute studies and do not reflect effects that can take time to develop, such as tardive dyskinesia, the long-term accumulation of body weight, and the development of insulin resistance/type 2 diabetes mellitus.40 The data presented are from carefully conducted registration trials that enrolled subjects who fulfilled restrictive inclusion/exclusion criteria. Such patients may differ from those encountered in routine clinical practice. Keep in mind that adverse events may differ in terms of impact and may not be clinically relevant if the adverse event is mild, time-limited, or easily managed. Moreover, different patients carry different propensities to experience different adverse events or to achieve a therapeutic response.
Continue to: Bottom Line