Pharmacologic profile, adverse reactions
Aripiprazole is a dopamine partial agonist atypical antipsychotic that has been commercially available in the United States since November 15, 2002, and its adverse effect profile is well characterized. The LAI formulation AL was approved on October 5, 2015. In the pivotal, 12-week, fixed-dose, placebo-controlled clinical trial of AL 441 mg or 882 mg monthly for adults with an acute exacerbation of schizophrenia, the only adverse effect that occurred in ≥5% of AL-treated patients and a rate at least twice that of placebo was akathisia (441 mg: 11%; 882 mg: 11%; placebo: 4%).10 Only 2 of 415 AL-treated patients discontinued the study due to akathisia. Injection-site reactions were reported by 4%, 5%, and 2% of patients treated with AL 441 mg, AL 882 mg, and placebo, respectively. Most of these were injection-site pain associated with the first injection, and decreased with each subsequent injection. Other injection-site reactions (induration, swelling, and redness) occurred at rates <1%.11
Having established that the range of plasma aripiprazole levels consistent with effective treatment is bounded by levels seen with AL 441 mg or 882 mg monthly, the FDA did not require additional efficacy studies for new AL doses provided that pharmacokinetic (PK) studies could demonstrate levels within the effective range. This is consistent with how new doses of other LAI antipsychotic preparations have been addressed in the past. For example, the 37.5 mg dose of risperidone microspheres was approved based on PK data, although the pivotal efficacy trials included doses of 25 mg, 50 mg, and 75 mg.12 Based on PK studies, AL doses of 662 mg monthly, 882 mg every 6 weeks, and 1,064 mg every 8 weeks were previously approved.13 The approval process for ALncd followed a similar pathway, and is based on PK results combined with tolerability data amassed during the PK studies. The package insert thus notes that in PK studies the safety profile of ALncd was generally consistent with that observed for AL (see Tolerability).
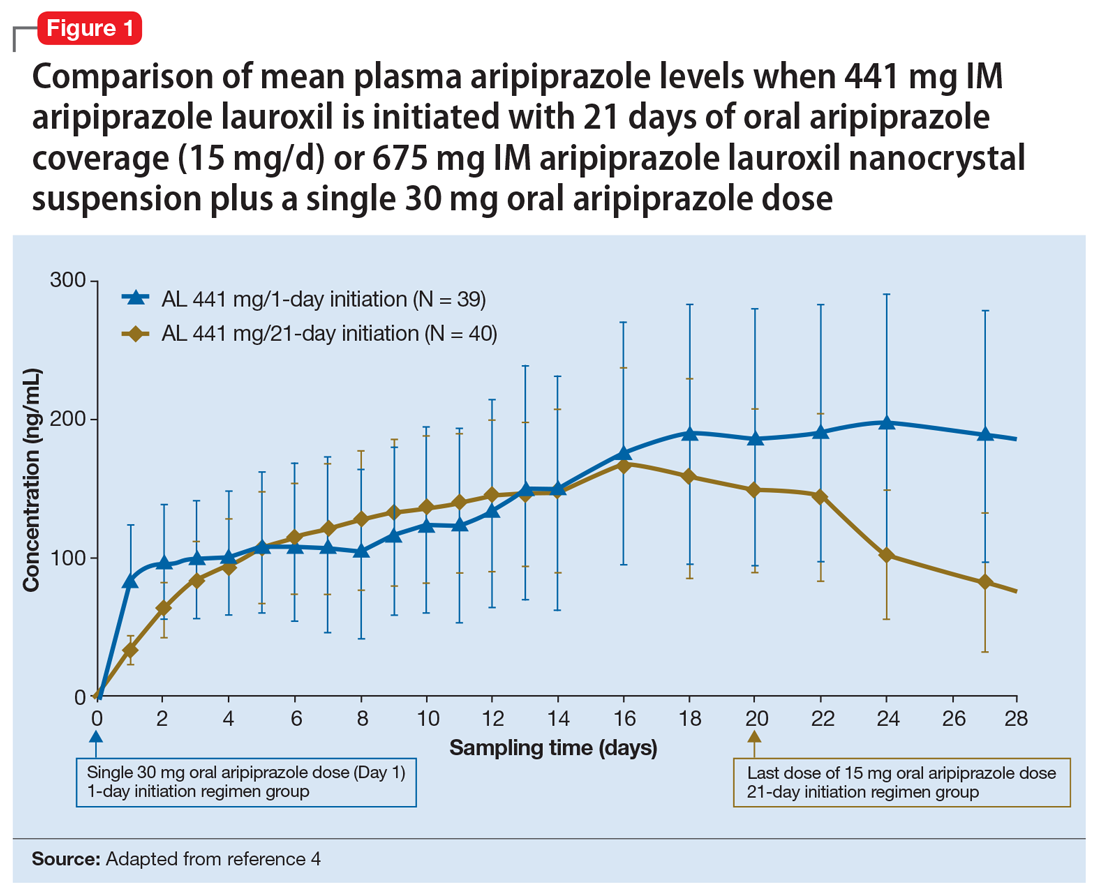
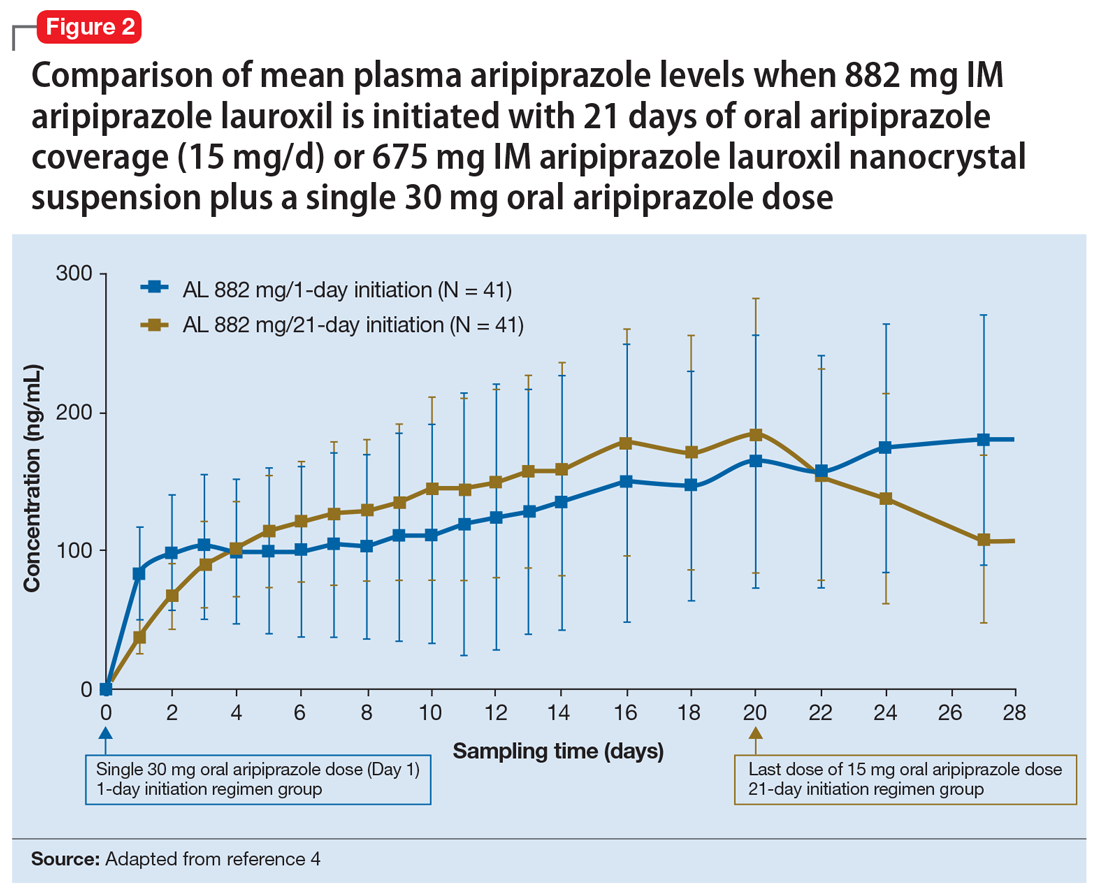
Pharmacokinetic outcomes. A comparative phase 1 PK study was performed to evaluate initiation regimens: either 21 days of oral aripiprazole (15 mg/d) and one AL dose (n = 81) or one injection of ALncd plus a single dose of 30 mg oral aripiprazole and one AL dose (n = 80). Patients were randomized 1:1:1:1 to receive an AL dose of either 441 mg or 882 mg combined with the oral or the new ALncdinitiation regimen. As shown in Figure 1 and Figure 2, the mean plasma levels seen with 675 mg IM ALncd plus a single dose of 30 mg oral aripiprazole were comparable with levels achieved using 21 days of oral aripiprazole coverage, regardless of whether the regimen was paired with a maintenance AL dose of 441 mg or 882 mg.4
Tolerability. In PK studies, the safety profile and incidences of injection site reactions of ALncd were generally consistent with those observed for aripiprazole lauroxil.9 In the phase I PK study comparing oral initiation with ALncd plus a single 30 mg oral aripiprazole dose, there were 2 mild cases of akathisia in the 21-day oral aripiprazole groups (n = 81) and 4 cases in the ALncd groups (n = 80) (3 mild cases, 1 moderate case). None of the adverse events related to akathisia were deemed serious, and no patients discontinued participation in the trial due to akathisia.9
Continue to: Clinical considerations