Suicidality. The risk of developing suicidal thoughts and behaviors with brexanolone is unknown, due to the relatively low number of exposures to brexanolone injection during clinical development and a mechanism of action distinct from that of existing antidepressant medications.3
Pharmacokinetics
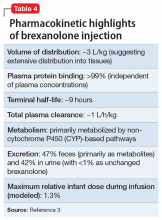
In clinical trials, brexanolone exhibited dose-proportional pharmacokinetics, and the terminal half-life is approximately 9 hours (Table 43). Brexanolone is metabolized by non-cytochrome P450 (CYP)-based pathways, including keto-reduction, glucuronidation, and sulfation.3 No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal or hepatic impairment, and no studies were conducted to evaluate the effects of other drugs on brexanolone.3
Lactation. A population pharmacokinetics model constructed from studies in the clinical development program calculated the maximum relative infant dose for brexanolone during infusion as 1.3%.3 Given the low oral bioavailability of brexanolone (<5%) in adults, the potential for breastfed infant exposure is considered low.3
Clinical considerations
Risk Evaluation and Mitigation Strategies (REMS) requirements. Brexanolone injection is a Schedule IV controlled substance. It has a “black-box” warning regarding excessive sedation and sudden loss of consciousness, which has been taken into account within the REMS drug safety program. Health care facilities and pharmacies must enroll in the REMS program and ensure that brexanolone is administered only to patients who are enrolled in the REMS program. Staff must be trained on the processes and procedures to administer brexanolone, and the facility must have a fall precautions protocol in place and be equipped with a programmable peristaltic IV infusion pump and continuous pulse oximetry with alarms.3
Monitoring. A REMS-trained clinician must be available continuously on-site to oversee each patient for the duration of the continuous IV infusion, which lasts 60 hours (2.5 days) and should be initiated early enough in the day to allow for recognition of excessive sedation. Patients must be monitored for hypoxia using continuous pulse oximetry equipped with an alarm and should also be assessed for excessive sedation every 2 hours during planned, non-sleep periods. If excessive sedation occurs, the infusion should be stopped until symptoms resolve, after which the infusion may be resumed at the same or a lower dose as clinically appropriate. In case of overdosage, the infusion should be stopped immediately and supportive measures initiated as necessary. Patients must not be the primary caregiver of dependents, and must be accompanied during interactions with their child(ren).
Continue to: Contraindications