Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
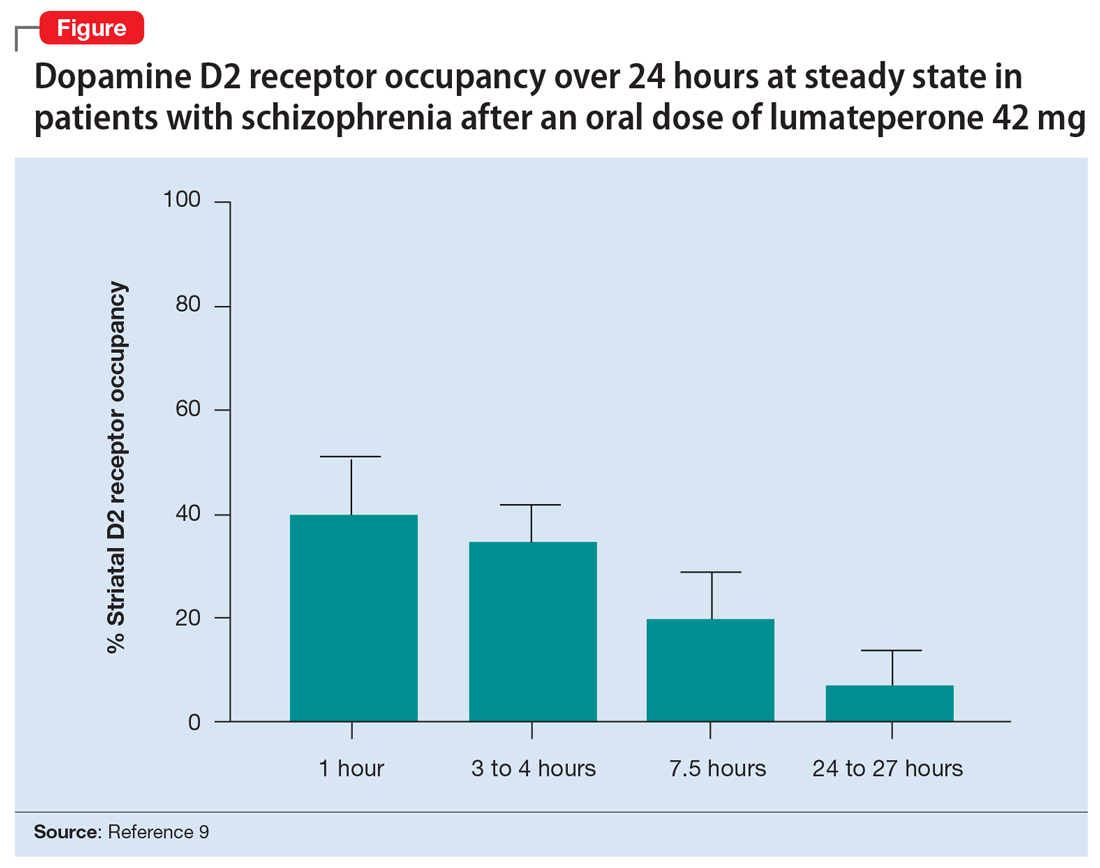
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9
How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-d-aspartate receptor subunits and glycogen synthase kinase 3 beta (GSK3B), properties that could impact cognition or negative symptoms in patients with schizophrenia.8
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...